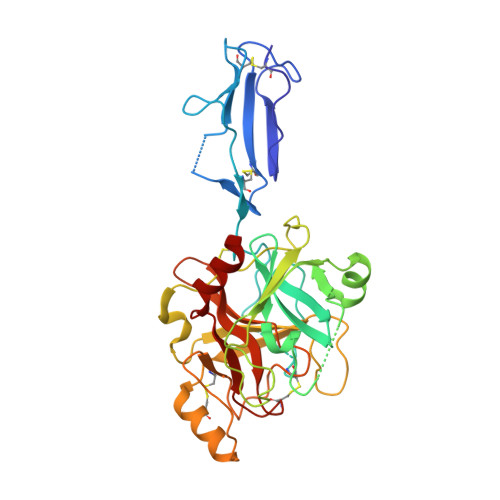
Monomeric structures of the zymogen and active catalytic domain of complement protease c1r: further insights into the c1 activation mechanism
Budayova-Spano, M., Grabarse, W., Thielens, N.M., Hillen, H., Lacroix, M., Schmidt, M., Fontecilla-Camps, J., Arlaud, G.J., Gaboriaud, C.(2002) Structure 10: 1509-1519
- PubMed: 12429092
- DOI: https://doi.org/10.1016/s0969-2126(02)00881-x
- Primary Citation of Related Structures:
1MD7, 1MD8 - PubMed Abstract:
C1r is the serine protease (SP) that mediates autoactivation of C1, the complex that triggers the classical complement pathway. We have determined the crystal structure of two fragments from the human C1r catalytic domain, each encompassing the second complement control protein (CCP2) module and the SP domain. The wild-type species has an active structure, whereas the S637A mutant is a zymogen. The structures reveal a restricted hinge flexibility of the CCP2-SP interface, and both are characterized by the unique alpha-helical conformation of loop E. The zymogen activation domain exhibits high mobility, and the active structure shows a restricted access to most substrate binding subsites. Further implications relevant to the C1r self-activation process are derived from protein-protein interactions in the crystals.
- LCCP, Institut de Biologie Structurale Jean-Pierre Ebel, CEA-CNRS, 41 rue Jules Horowitz, Grenoble, France.
Organizational Affiliation: