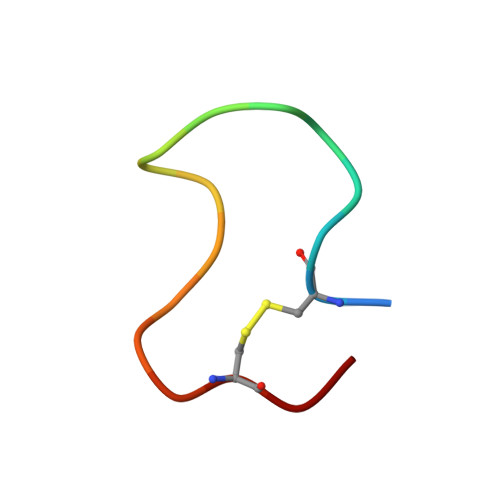
Comparison of NMR solution structures of the receptor binding domains of Pseudomonas aeruginosa pili strains PAO, KB7, and PAK: implications for receptor binding and synthetic vaccine design.
Campbell, A.P., McInnes, C., Hodges, R.S., Sykes, B.D.(1995) Biochemistry 34: 16255-16268
- PubMed: 8845350
- DOI: https://doi.org/10.1021/bi00050a005
- Primary Citation of Related Structures:
1KB7, 1KB8, 1NIL, 1NIM, 1PAN, 1PAO - PubMed Abstract:
The solution structures of peptide antigens from the receptor binding domains of Pseudomonas aeruginosa strains PAO and KB7 have been determined using two-dimensional 1H NMR techniques. Ensembles of solution conformations for the trans forms of these 17-residue disulfide-bridged peptides have been generated using a simulated annealing procedure in conjunction with distance and torsion angle restraints derived from NMR data. Comparison of the NMR-derived solution structures of the PAO and KB7 peptides, with that previously determined (McInnes et al., 1993) and herein refined for the PAK peptide reveals a common structural motif. All three peptide structures contain a type I beta-turn in the conserved sequence Asp134-X-X-Phe137 and a type II beta-turn in the conserved sequence Pro139-X-Gly-Cys142. However, the overall folds of the three peptides differ as well as the disposition of the side chains comprising the hydrophobic pockets. The similarities and differences between the structures of the three strains which bind to a common cell surface receptor are discussed in light of their contributions to synthetic vaccine design.
- Protein Engineering Network of Centers of Excellence, University of Alberta, Edmonton, Canada.
Organizational Affiliation: