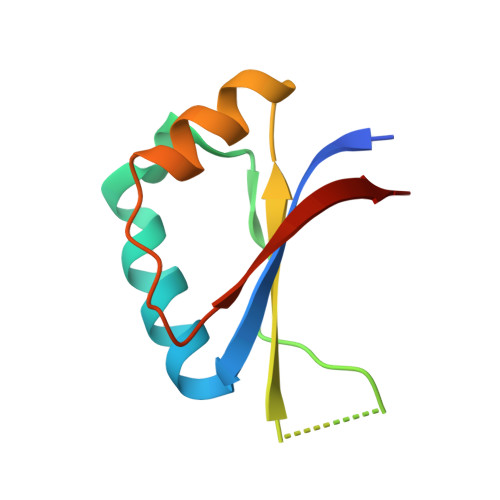
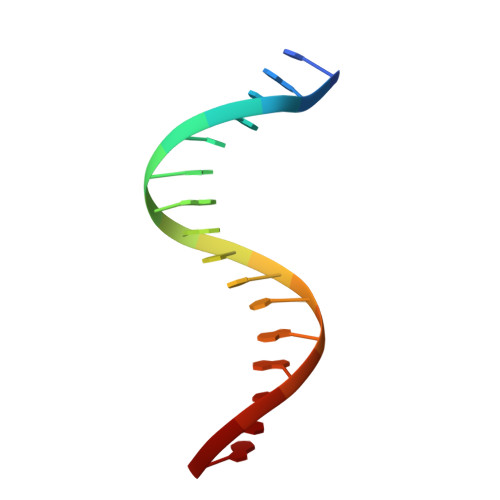
The structural basis of DNA target discrimination by papillomavirus E2 proteins.
Kim, S.S., Tam, J.K., Wang, A.F., Hegde, R.S.(2000) J Biological Chem 275: 31245-31254
- PubMed: 10906136
- DOI: https://doi.org/10.1074/jbc.M004541200
- Primary Citation of Related Structures:
1F9F, 1JJ4 - PubMed Abstract:
The papillomavirus E2 proteins regulate the transcription of all papillomavirus genes and are necessary for viral DNA replication. Disruption of the E2 gene is commonly associated with malignancy in cervical carcinoma, indicating that E2 has a role in regulating tumor progression. Although the E2 proteins from all characterized papillomaviruses bind specifically to the same 12-base pair DNA sequence, the cancer-associated human papillomavirus E2 proteins display a unique ability to detect DNA flexibility and intrinsic curvature. To understand the structural basis for this phenomenon, we have determined the crystal structures of the human papillomavirus-18 E2 DNA-binding domain and its complexes with high and low affinity binding sites. The E2 protein is a dimeric beta-barrel and the E2-DNA interaction is accompanied by a large deformation of the DNA as it conforms to the E2 surface. DNA conformation and E2-DNA contacts are similar in both high and low affinity complexes. The differences in affinity correlate with the flexibility of the DNA sequence. Preferences of E2 proteins from different papillomavirus strains for flexible or prevent DNA targets correlate with the distribution of positive charge on their DNA interaction surfaces, suggesting a role for electrostatic forces in the recognition of DNA deformability.
- Department of Biochemistry and Program in Structural Biology, New York University Medical Center, Skirball Institute of Biomolecular Medicine, New York, New York 10016, USA.
Organizational Affiliation: