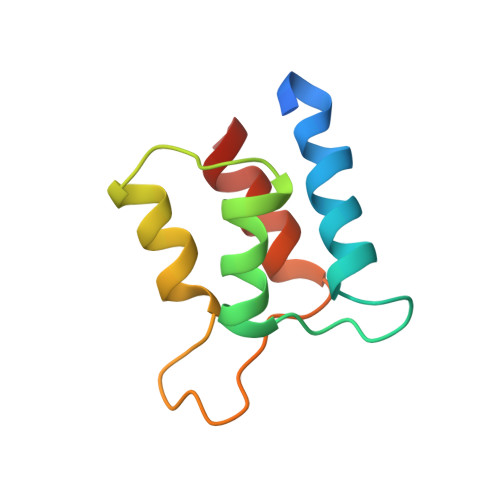
Solution structure and backbone dynamics of the defunct domain of calcium vector protein.
Theret, I., Baladi, S., Cox, J.A., Gallay, J., Sakamoto, H., Craescu, C.T.(2001) Biochemistry 40: 13888-13897
- PubMed: 11705378
- Primary Citation of Related Structures:
1J7Q, 1J7R - PubMed Abstract:
CaVP (calcium vector protein) is a Ca(2+) sensor of the EF-hand protein family which is highly abundant in the muscle of Amphioxus. Its three-dimensional structure is not known, but according to the sequence analysis, the protein is composed of two domains, each containing a pair of EF-hand motifs. We determined recently the solution structure of the C-terminal domain (Trp81-Ser161) and characterized the large conformational and dynamic changes induced by Ca(2+) binding. In contrast, the N-terminal domain (Ala1-Asp86) has lost the capacity to bind the metal ion due to critical mutations and insertions in the two calcium loops. In this paper, we report the solution structure of the N-terminal domain and its backbone dynamics based on NMR spectroscopy, nuclear relaxation, and molecular modeling. The well-resolved three-dimensional structure is typical of a pair of EF-hand motifs, joined together by a short antiparallel beta-sheet. The tertiary arrangement of the two EF-hands results in a closed-type conformation, with near-antiparallel alpha-helices, similar to other EF-hand pairs in the absence of calcium ions. To characterize the internal dynamics of the protein, we measured the (15)N nuclear relaxation rates and the heteronuclear NOE effect in (15)N-labeled N-CaVP at a magnetic field of 11.74 T and 298 K. The domain is mainly monomeric in solution and undergoes an isotropic Brownian rotational diffusion with a correlation time of 7.1 ns, in good agreement with the fluorescence anisotropy decay measurements. Data analysis using a model-free procedure showed that the amide backbone groups in the alpha-helices and beta-strands undergo highly restricted movements on a picosecond to nanosecond time scale. The amide groups in Ca(2+) binding loops and in the linker fragment also display rapid fluctuations with slightly increased amplitudes.
- INSERM U350 and Institut Curie-Recherche, Centre Universitaire, Bâtiments 110-112, 91405 Orsay Cedex, France.
Organizational Affiliation: