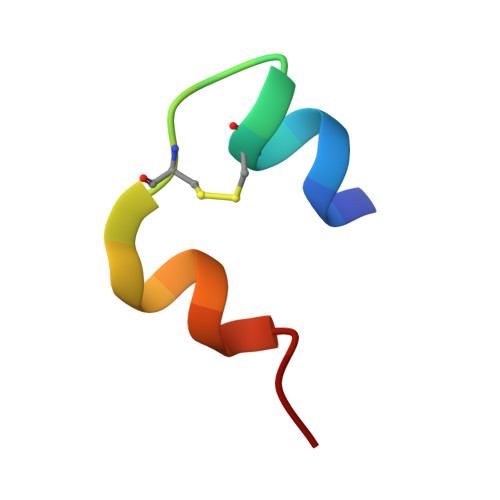
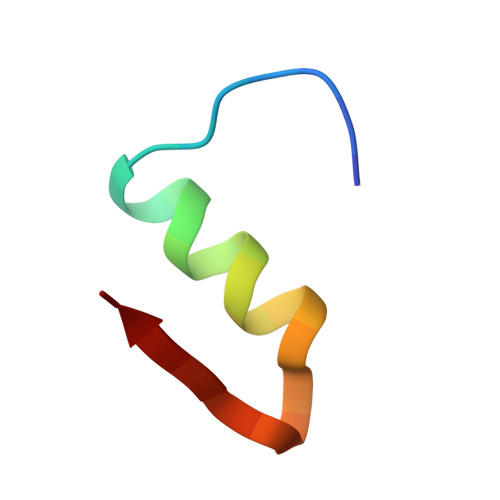
Crystal structure of destripeptide (B28-B30) insulin: implications for insulin dissociation.
Ye, J., Chang, W., Liang, D.(2001) Biochim Biophys Acta 1547: 18-25
- PubMed: 11343787
- DOI: https://doi.org/10.1016/s0167-4838(01)00160-1
- Primary Citation of Related Structures:
1HTV - PubMed Abstract:
Destripeptide (B28-B30) insulin (DTRI) is an insulin analogue that has much weaker association ability than native insulin but keeps most of its biological activity. It can be crystallized from a solution containing zinc ions at near-neutral pH. Its crystal structure has been determined by molecular replacement and refined at 1.9 A resolution. DTRI in the crystal exists as a loose hexamer compared with 2Zn insulin. The hexamer only contains one zinc ion that coordinates to the B10 His residues of three monomers. Although residues B28-B30 are located in the monomer-monomer interface within a dimer, the removal of them can simultaneously weaken both the interactions between monomers within the dimer and the interactions between dimers. Because the B-chain C-terminus of insulin is very flexible, we take the DTRI hexamer as a transition state in the native insulin dissociation process and suggest a possible dissociation process of the insulin hexamer based on the DTRI structure.
- National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, 15 Datun Road, Chaoyang District, 100101, Beijing, PR China.
Organizational Affiliation: