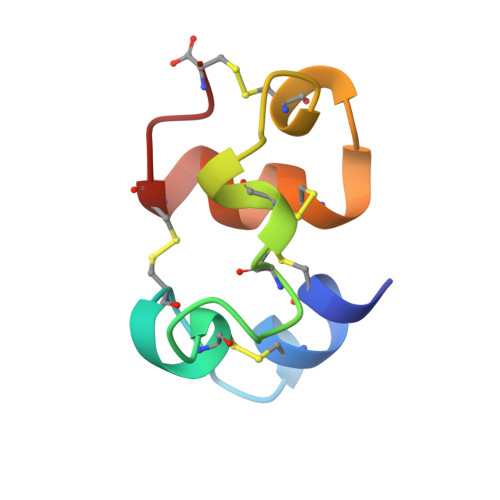
NMR Structure of the Euplotes Raikovi Pheromone Er- 23 and Identification of its Five Disulfide Bonds
Zahn, R., Damberger, F., Ortenzi, C., Luporini, P., Wuthrich, K.(2001) J Mol Biology 313: 923
- PubMed: 11700049
- DOI: https://doi.org/10.1006/jmbi.2001.5099
- Primary Citation of Related Structures:
1HA8 - PubMed Abstract:
The NMR solution structure of the 51 residue pheromone Er-23 from the ciliated protozoan Euplotes raikovi (Er) was calculated with the torsion angle dynamics program DYANA from 582 nuclear Overhauser enhancement (NOE) upper limit distance constraints, 46 dihedral angle constraints and 30 disulfide bond constraints. The disulfide bridges had not been assigned by chemical methods, and initially were assigned tentatively on the basis of inspection of the positioning of the Cys sulfhydryl groups in a bundle of 20 conformers that was calculated without disulfide bond constraints. The assignment of disulfide bridges was then validated by structure calculations that assessed the compatibility of plausible alternative Cys-Cys disulfide combinations with the input of NOE upper distance constraints and dihedral angle constraints. For a group of 20 conformers used to characterize the solution structure, the average pairwise root-mean-square distances from the mean coordinates calculated for the backbone heavy atoms N, C(alpha) and C' of resideus 1-51 is 0.38 A. The molecular architecture consists of a three-dimensional arrangement of five helices comprised of residues 2-8, 14-17, 26-29, 34-36 and 38-47, with five disulfide bridges in the positions 3-24, 6-16, 13-47, 27-40, and 35-51, which has so far not been represented in the Protein Data Bank. Er-23 is unique among presently known Er-pheromones with respect to size, sequence, the number of disulfide bonds and the three-dimensional structure, thus providing a new structural basis for rationalizing the physiological functions of this protein family.
- Institut für Molekularbiologie und Biophysik, Eidgenössische Technische Hochschule, Zürich, CH-8093, Switzerland
Organizational Affiliation: