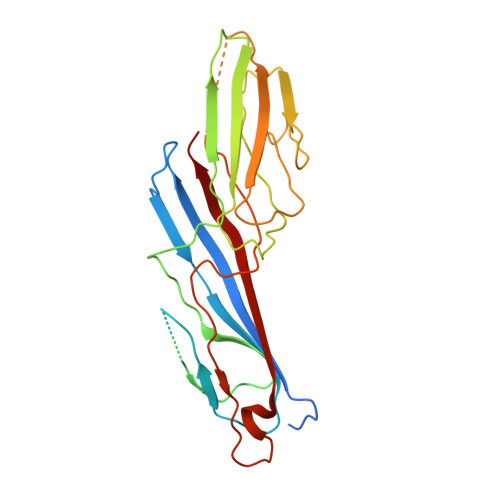
Study of the Interaction of the Medium Chain Mu2 Subunit of the Clathrin-Associated Adapter Protein Complex 2 with Cytotoxic T-Lymphocyte Antigen 4 and Cd28
Follows, E.R., Mcpheat, J.C., Minshull, C., Moore, N.C., Pauptit, R.A., Rowsell, S., Stacey, C.L., Stanway, J.J., Taylor, I.W., Abbott, W.M.(2001) Biochem J 359: 427
- PubMed: 11583591
- DOI: https://doi.org/10.1042/0264-6021:3590427
- Primary Citation of Related Structures:
1H6E - PubMed Abstract:
The medium chain mu 2 subunit (AP50) of the clathrin-associated adapter protein complex 2 (AP-2) interacts specifically with the tyrosine-based signals of several integral membrane proteins through the consensus sequence YXXPhi, where X can be any residue and Phi is a large hydrophobic residue. Using surface plasmon resonance combined with structural information, we have analysed the interaction of AP50 with peptides derived from the cytoplasmic tail of cytotoxic T-lymphocyte antigen 4 (CTLA-4). The crystal structure of AP50 in complex with a CTLA-4-derived peptide was determined to 3.6 A (1 A=0.1 nm) resolution. The binding domain of AP50 (residues 164-435) was expressed in Escherichia coli and purified. In agreement with previous reports, the AP50 domain bound to residues 152-174 of CTLA-4, but not to the same peptide that was phosphorylated at the single tyrosine residue (position 165). The interaction exhibited fast kinetics with rapid on and off rates and a K(d) of 0.7 microM. In order to further understand why AP50 binds to CTLA-4, but not to the homologous receptor CD28, a comparison of binding of AP50 with five peptides with single changes in and around the YXXPhi motif to the equivalent residues of CD28 was made. T162H greatly reduced binding, whereas T161L had little effect. Mutations G163S, V164D and K167N all exhibited reduced binding. Modelling of the single amino acid changes using structural information, was in broad agreement with the binding data, demonstrating that residues outside of the YXXPhi motif are also important in the interaction of membrane proteins with AP50.
- Enabling Science and Technology, Biology Department, AstraZeneca, Mereside, Alderley Park, Macclesfield, Cheshire SK10 4TG, UK.
Organizational Affiliation: