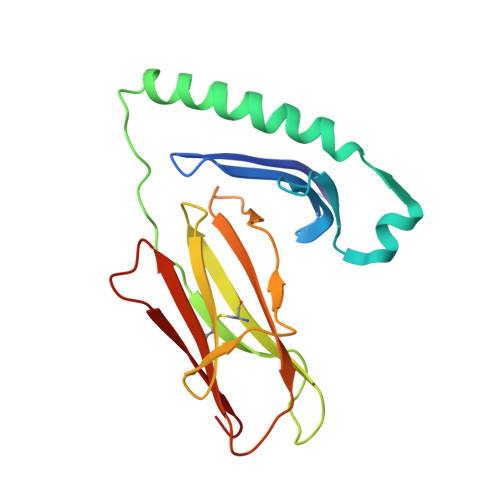
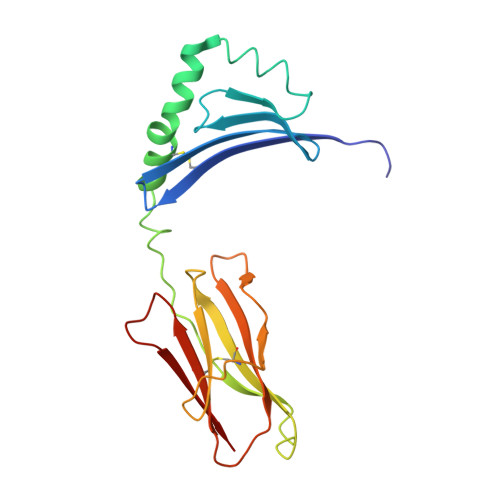
A Functional and Structural Basis for Tcr Cross-Reactivity in Multiple Sclerosis
Lang, H., Jacobsen, H., Ikemizu, S., Andersson, C., Harlos, K., Madsen, L., Hjorth, P., Sondergaard, L., Svejgaard, A., Wucherpfennig, K., Stuart, D.I., Bell, J.I., Jones, E.Y., Fugger, L.(2002) Nat Immunol 3: 940
- PubMed: 12244309
- DOI: https://doi.org/10.1038/ni835
- Primary Citation of Related Structures:
1H15 - PubMed Abstract:
The multiple sclerosis (MS)-associated HLA major histocompatibility complex (MHC) class II alleles DRB1*1501, DRB5*0101 and DQB1*0602 are in strong linkage disequilibrium, making it difficult to determine which is the principal MS risk gene. Here we show that together the DRB1 and DRB5 loci may influence susceptibility to MS. We demonstrate that a T cell receptor (TCR) from an MS patient recognized both a DRB1*1501-restricted myelin basic protein (MBP) and DRB5*0101-restricted Epstein-Barr virus (EBV) peptide. Crystal structure determination of the DRB5*0101-EBV peptide complex revealed a marked degree of structural equivalence to the DRB1*1501-MBP peptide complex at the surface presented for TCR recognition. This provides structural evidence for molecular mimicry involving HLA molecules. The structural details suggest an explanation for the preponderance of MHC class II associations in HLA-associated diseases.
- Nuffield Department of Clinical Medicine, John Radcliffe Hospital, Headington, Oxford OX3 9DU, UK.
Organizational Affiliation: