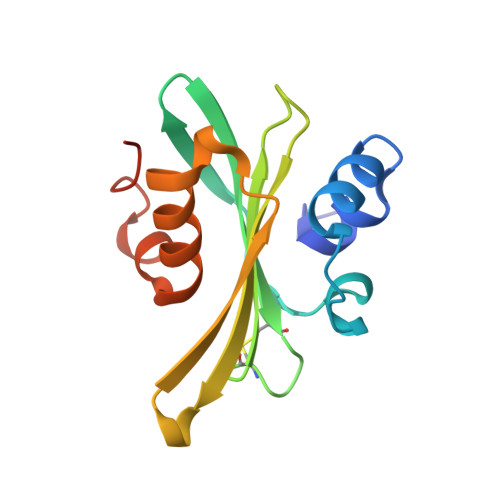
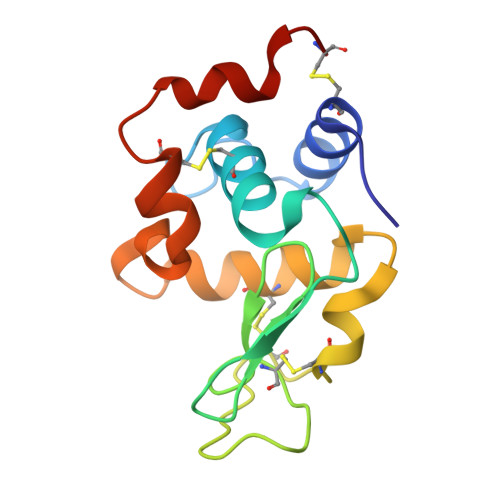
Structure and Evolution of the Ivy Protein Family, Unexpected Lysozyme Inhibitors in Gram-Negative Bacteria.
Abergel, C., Monchois, V., Byrne, D., Chenivesse, S., Lembo, F., Lazzaroni, J., Claverie, J.(2007) Proc Natl Acad Sci U S A 104: 6394
- PubMed: 17405861
- DOI: https://doi.org/10.1073/pnas.0611019104
- Primary Citation of Related Structures:
1GPQ, 1UUZ, 1XS0 - PubMed Abstract:
Part of an ancestral bactericidal system, vertebrate C-type lysozyme targets the peptidoglycan moiety of bacterial cell walls. We report the crystal structure of a protein inhibitor of C-type lysozyme, the Escherichia coli Ivy protein, alone and in complex with hen egg white lysozyme. Ivy exhibits a novel fold in which a protruding five-residue loop appears essential to its inhibitory effect. This feature guided the identification of Ivy orthologues in other Gram-negative bacteria. The structure of the evolutionary distant Pseudomonas aeruginosa Ivy orthologue was also determined in complex with hen egg white lysozyme, and its antilysozyme activity was confirmed. Ivy expression protects porous cell-wall E. coli mutants from the lytic effect of lysozyme, suggesting that it is a response against the permeabilizing effects of the innate vertebrate immune system. As such, Ivy acts as a virulence factor for a number of Gram-negative bacteria-infecting vertebrates.
- Structural and Genomic Information Laboratory, Centre National de la Recherche Scientifique, Unité Propre de Recherche 2589, 13288 Marseille, Cedex 9, France. chantal.abergel@igs.cnrs-mrs.fr
Organizational Affiliation: