The interaction of thrombin with fibrinogen. A structural basis for its specificity.
Stubbs, M.T., Oschkinat, H., Mayr, I., Huber, R., Angliker, H., Stone, S.R., Bode, W.(1992) Eur J Biochem 206: 187-195
- PubMed: 1587268
- DOI: https://doi.org/10.1111/j.1432-1033.1992.tb16916.x
- Primary Citation of Related Structures:
1FPH - PubMed Abstract:
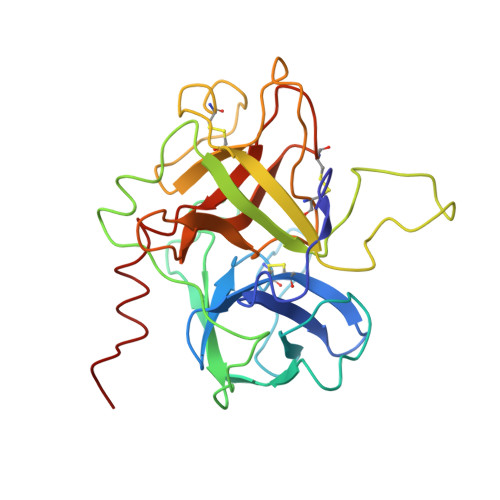
The structure of the ternary complex of human alpha-thrombin with a covalently bound analogue of fibrinopeptide A and a C-terminal hirudin peptide has been determined by X-ray diffraction methods at 0.25 nm resolution. Fibrinopeptide A folds in a compact manner, bringing together hydrophobic residues that slot into the apolar binding site of human alpha-thrombin. Fibrinogen residue Phe8 occupies the aryl-binding site of thrombin, adjacent to fibrinogen residues Leu9 and Val15 in the S2 subsite. The species diversity of fibrinopeptide A is analysed with respect to its conformation and its interaction with thrombin. The non-covalently attached peptide fragment hirudin(54-65) exhibits an identical conformation to that observed in the hirudin-thrombin complex. The occupancy of the secondary fibrinogen-recognition exosite by this peptide imposes restrictions on the manner of fibrinogen binding. The surface topology of the thrombin molecule indicates positions P1'-P3', differ from those of the canonical serine-proteinase inhibitors, suggesting a mechanical model for the switching of thrombin activity from fibrinogen cleavage to protein-C activation on thrombomodulin complex formation. The multiple interactions between thrombin and fibrinogen provide an explanation for the narrow specificity of thrombin. Structural grounds can be put forward for certain congenital clotting disorders.
- Max-Planck-Institut für Biochemie, Martinsried bei München, Federal Republic of Germany.
Organizational Affiliation: