The structural basis for spectral variations in green fluorescent protein.
Palm, G.J., Zdanov, A., Gaitanaris, G.A., Stauber, R., Pavlakis, G.N., Wlodawer, A.(1997) Nat Struct Biol 4: 361-365
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
(1997) Nat Struct Biol 4: 361-365
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| GREEN FLUORESCENT PROTEIN | 237 | Aequorea victoria | Mutation(s): 3 Gene Names: GFP | 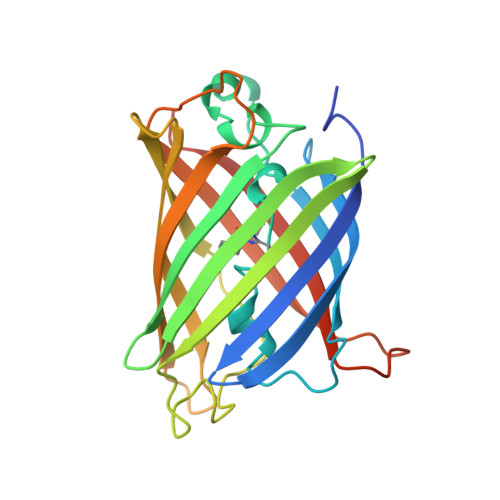 | |
UniProt | |||||
Find proteins for P42212 (Aequorea victoria) Explore P42212 Go to UniProtKB: P42212 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P42212 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| CCY Query on CCY | A | L-PEPTIDE LINKING | C14 H19 N3 O4 S |  | CYS, TYR, GLY |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 89.8 | α = 90 |
| b = 89.8 | β = 90 |
| c = 130.1 | γ = 120 |
| Software Name | Purpose |
|---|---|
| AMoRE | phasing |
| X-PLOR | refinement |
| DENZO | data reduction |
| SCALEPACK | data scaling |