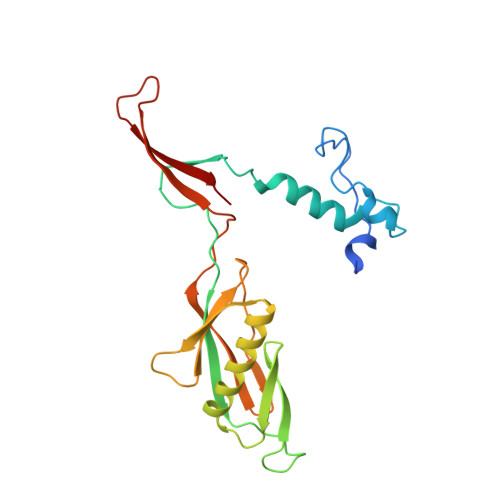
Structure of bacteriophage T4 gene product 11, the interface between the baseplate and short tail fibers.
Leiman, P.G., Kostyuchenko, V.A., Shneider, M.M., Kurochkina, L.P., Mesyanzhinov, V.V., Rossmann, M.G.(2000) J Mol Biology 301: 975-985
- PubMed: 10966799
- DOI: https://doi.org/10.1006/jmbi.2000.3989
- Primary Citation of Related Structures:
1EL6 - PubMed Abstract:
Bacteriophage T4, like all other viruses, is required to be stable while being transmitted from host to host, but also is poised to eject efficiently and rapidly its double-stranded DNA genome to initiate infection. The latter is coordinated by the recognition of receptors on Escherichia coli cells by the long tail fibers and subsequent irreversible attachment by the short tail fibers. These fibers are attached to the baseplate, a multi-subunit assembly at the distal end of the tail. Recognition and attachment induce a conformational transition of the baseplate from a hexagonal to a star-shaped structure. The crystal structure of gene product 11 (gp11), a protein that connects the short tail fibers to the baseplate, has been determined to 2.0 A resolution using multiple wavelength anomalous dispersion with Se. This structure is compared to the trimeric structure of gp9, which connects the baseplate with the long tail fibers. The structure of gp11 is a trimer with each monomer consisting of 218 residues folded into three domains. The N-terminal domains form a central, trimeric, parallel coiled coil surrounded by the middle "finger" domains. The fingers emanate from the carboxy-terminal beta-annulus domain, which, by comparison with the T4 whisker "fibritin" protein, is probably responsible for trimerization. The events leading from recognition of the host to the ejection of viral DNA must be communicated along the assembled trimeric (gp9)(3) attached to the long tail fibers via the trimeric baseplate protein (gp10)(3) to the trimeric (gp11)(3) and the trimeric short tail fibers.
- Department of Biological Sciences, Purdue University, West Lafayette, IN, 47907-1392, USA.
Organizational Affiliation: