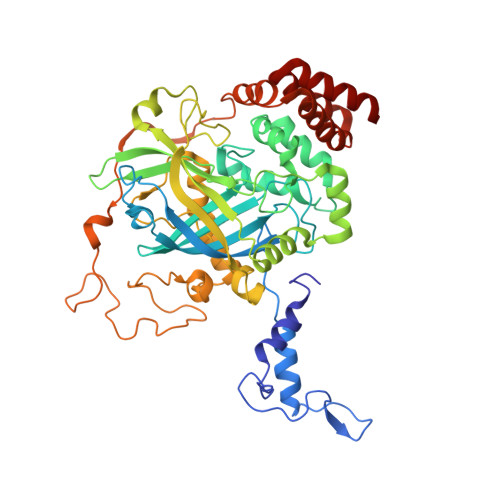
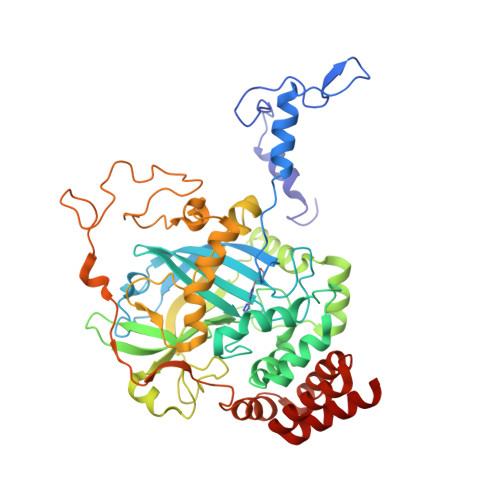
Active and inhibited human catalase structures: ligand and NADPH binding and catalytic mechanism.
Putnam, C.D., Arvai, A.S., Bourne, Y., Tainer, J.A.(2000) J Mol Biology 296: 295-309
- PubMed: 10656833
- DOI: https://doi.org/10.1006/jmbi.1999.3458
- Primary Citation of Related Structures:
1DGB, 1DGF, 1DGG, 1DGH - PubMed Abstract:
Human catalase is an heme-containing peroxisomal enzyme that breaks down hydrogen peroxide to water and oxygen; it is implicated in ethanol metabolism, inflammation, apoptosis, aging and cancer. The 1. 5 A resolution human enzyme structure, both with and without bound NADPH, establishes the conserved features of mammalian catalase fold and assembly, implicates Tyr370 as the tyrosine radical, suggests the structural basis for redox-sensitive binding of cognate mRNA via the catalase NADPH binding site, and identifies an unexpectedly substantial number of water-mediated domain contacts. A molecular ruler mechanism based on observed water positions in the 25 A-long channel resolves problems for selecting hydrogen peroxide. Control of water-mediated hydrogen bonds by this ruler selects for the longer hydrogen peroxide and explains the paradoxical effects of mutations that increase active site access but lower catalytic rate. The heme active site is tuned without compromising peroxide binding through a Tyr-Arg-His-Asp charge relay, arginine residue to heme carboxylate group hydrogen bonding, and aromatic stacking. Structures of the non-specific cyanide and specific 3-amino-1,2, 4-triazole inhibitor complexes of human catalase identify their modes of inhibition and help reveal the catalytic mechanism of catalase. Taken together, these resting state and inhibited human catalase structures support specific, structure-based mechanisms for the catalase substrate recognition, reaction and inhibition and provide a molecular basis for understanding ethanol intoxication and the likely effects of human polymorphisms.
- Department of Molecular Biology, Skaggs Institute for Chemical Biology, The Scripps Research Institute, MB 4, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA.
Organizational Affiliation: