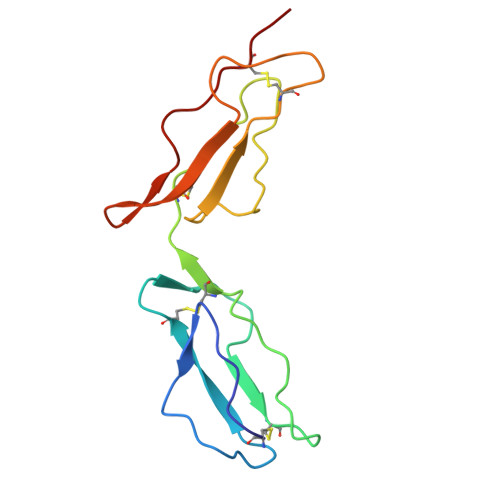
Crystal structure of two CD46 domains reveals an extended measles virus-binding surface.
Casasnovas, J.M., Larvie, M., Stehle, T.(1999) EMBO J 18: 2911-2922
- PubMed: 10357804
- DOI: https://doi.org/10.1093/emboj/18.11.2911
- Primary Citation of Related Structures:
1CKL - PubMed Abstract:
Measles virus is a paramyxovirus which, like other members of the family such as respiratory syncytial virus, is a major cause of morbidity and mortality worldwide. The cell surface receptor for measles virus in humans is CD46, a complement cofactor. We report here the crystal structure at 3.1 A resolution of the measles virus-binding fragment of CD46. The structure reveals the architecture and spatial arrangement of two glycosylated short consensus repeats with a pronounced interdomain bend and some flexibility at the domain interface. Amino acids involved in measles virus binding define a large, glycan-free surface that extends from the top of the first to the bottom of the second repeat. The extended virus-binding surface of CD46 differs strikingly from those reported for the human virus receptor proteins CD4 and intercellular cell adhesion molecule-1 (ICAM-1), suggesting that the CD46 structure utilizes a novel mode of virus recognition. A highly hydrophobic and protruding loop at the base of the first repeat bears a critical virus-binding residue, thereby defining an important recognition epitope. Molecules that mimic the conformation of this loop potentially could be effective anti-viral agents by preventing binding of measles virus to CD46.
- Department of Biosciences at NOVUM, Karolinska Institute, 14157 Huddinge, Sweden.
Organizational Affiliation: