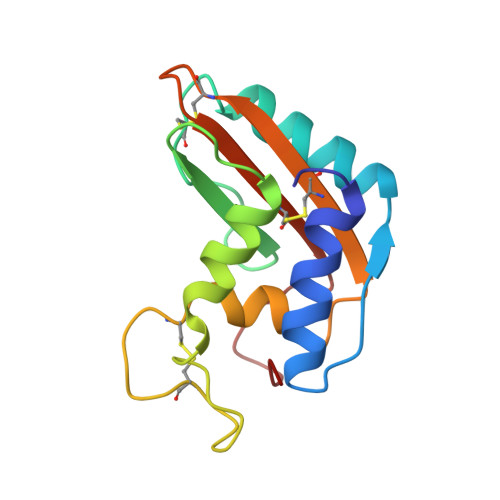
NMR solution structure of the pathogenesis-related protein P14a.
Fernandez, C., Szyperski, T., Bruyere, T., Ramage, P., Mosinger, E., Wuthrich, K.(1997) J Mol Biology 266: 576-593
- PubMed: 9067611
- DOI: https://doi.org/10.1006/jmbi.1996.0772
- Primary Citation of Related Structures:
1CFE - PubMed Abstract:
The nuclear magnetic resonance (NMR) structure of the 15 kDa pathogenesis-related protein P14a, which displays antifungicidal activity and is induced in tomato leaves as a response to pathogen infection, was determined using 15N/13C doubly labeled and unlabeled protein samples. In all, 2030 conformational constraints were collected as input for the distance geometry program DIANA. After energy-minimization with the program OPAL the 20 best conformers had an average root-mean-square deviation value relative to the mean coordinates of 0.88 A for the backbone atoms N, C(alpha) and C', and 1.30 A for all heavy atoms. P14a contains four alpha-helices (I to IV) comprising residues 4 to 17, 27 to 40, 64 to 72 and 93 to 98, a short 3(10)-helix of residues 73 to 75 directly following helix III, and a mixed, four-stranded beta-sheet with topology +3x, -2x, +1, containing the residues 24-25, 53 to 58, 104 to 111 and 117 to 124. These regular secondary structure elements form a novel, complex alpha + beta topology in which the alpha-helices I, III and IV and the 3(10)-helix are located above the plane defined by the beta-sheet, and the alpha-helix II lies below this plane. The alpha-helices and beta-strands are thus arranged in three stacked layers, which are stabilized by two distinct hydrophobic cores associated with the two layer interfaces, giving rise to an "alpha-beta-alpha sandwich". The three-dimensional structure of P14a provides initial leads for identification of the so far unknown active sites and the mode of action of the protein, which is of direct interest for the generation of transgenic plants with improved host defense properties.
- Institut für Molekularbiologie und Biophysik, Eidgenössische Technische Hochschule-Hönggerberg, Zürich, Switzerland.
Organizational Affiliation: