Highly selective inhibitors of protein kinases CLK and HIPK with the furo[3,2-b]pyridine core.
Nemec, V., Maier, L., Berger, B.T., Chaikuad, A., Drapela, S., Soucek, K., Knapp, S., Paruch, K.(2021) Eur J Med Chem 215: 113299-113299
- PubMed: 33636538
- DOI: https://doi.org/10.1016/j.ejmech.2021.113299
- Primary Citation of Related Structures:
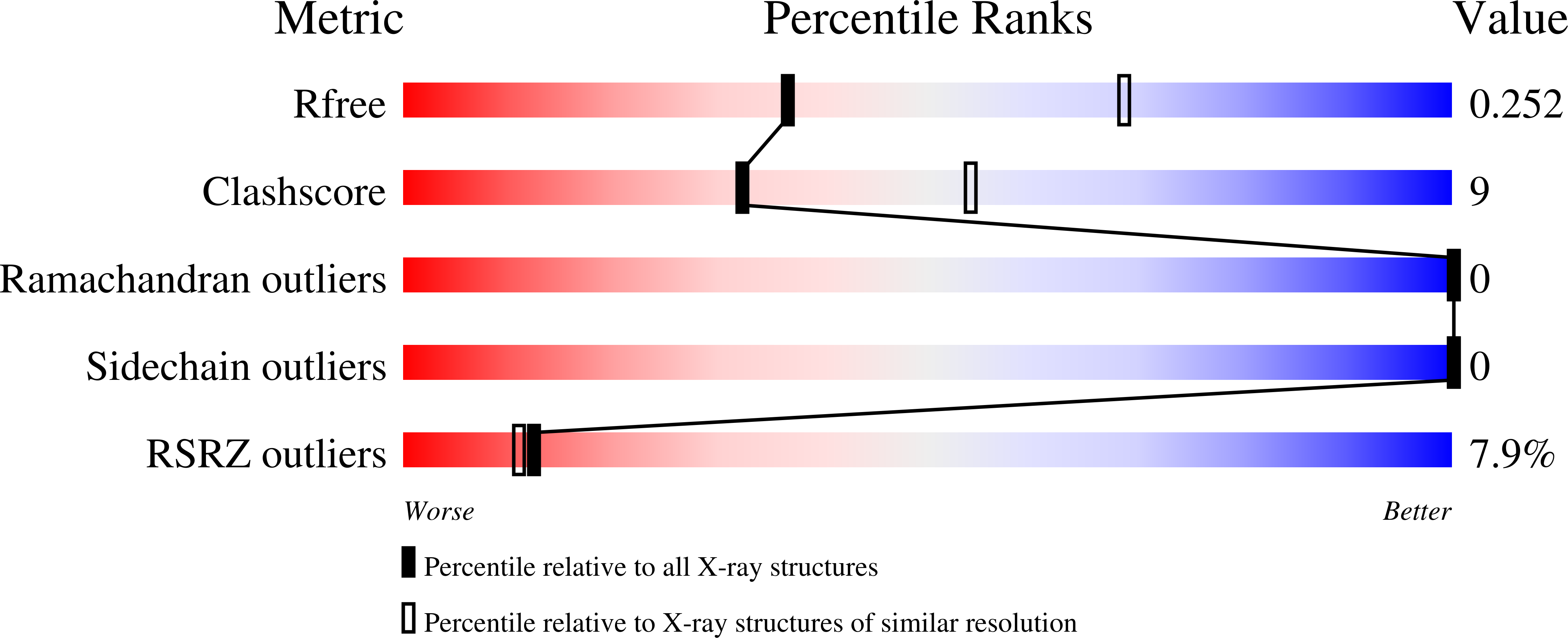
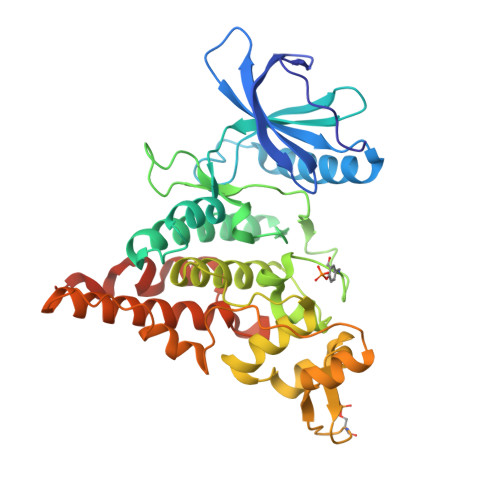
7NCF - PubMed Abstract:
The furo [3,2-b]pyridine motif represents a relatively underexplored central pharmacophore in the area of kinase inhibitors. Herein, we report flexible synthesis of 3,5-disubstituted furo [3,2-b]pyridines that relies on chemoselective couplings of newly prepared 5-chloro-3-iodofuro [3,2-b]pyridine. This methodology allowed efficient second-generation synthesis of the state-of-the-art chemical biology probe for CLK1/2/4 MU1210, and identification of the highly selective inhibitors of HIPKs MU135 and MU1787 which are presented and characterized in this study, including the X-ray crystal structure of MU135 in HIPK2. chemical biology probe.
Organizational Affiliation:
Department of Chemistry, Faculty of Science, Masaryk University, Kamenice 5, 625 00, Brno, Czech Republic; International Clinical Research Center, Center for Biomolecular and Cellular Engineering, St. Anne's University Hospital in Brno, Pekařská 53, 656 91, Brno, Czech Republic.