An Uncommon Type II PKS Catalyzes Biosynthesis of Aryl Polyene Pigments.
Grammbitter, G.L.C., Schmalhofer, M., Karimi, K., Shi, Y.M., Schoner, T.A., Tobias, N.J., Morgner, N., Groll, M., Bode, H.B.(2019) J Am Chem Soc 141: 16615-16623
- PubMed: 30908039
- DOI: https://doi.org/10.1021/jacs.8b10776
- Primary Citation of Related Structures:
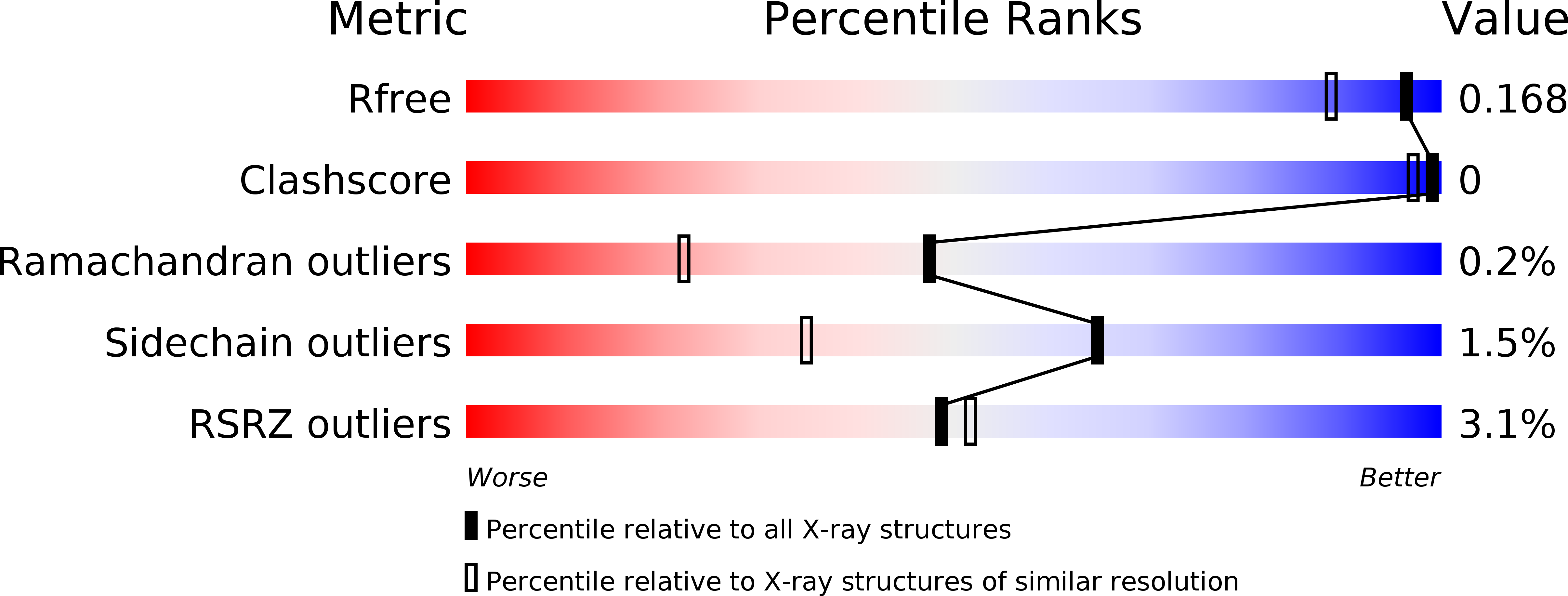
6QSP, 6QSR - PubMed Abstract:
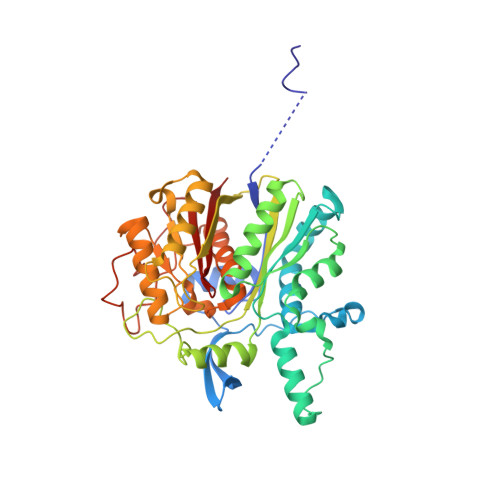
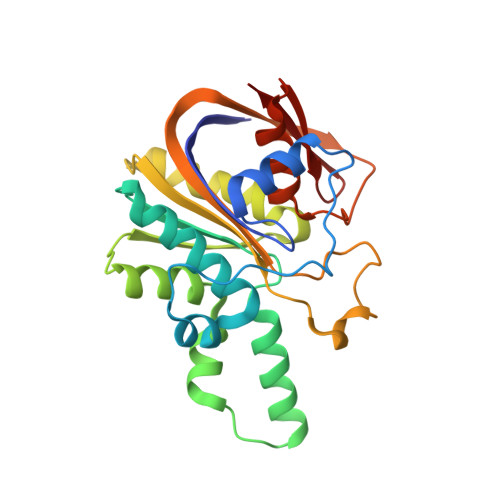
Aryl polyene (APE) pigments are a widely distributed class of bacterial polyketides. So far, little is known about the biosynthesis of these compounds, which are produced by a novel type II polyketide synthase (PKS). We have identified all enzymes involved in APE biosynthesis and determined their peculiar functions. The biosynthesis was reconstituted in vitro , and ACP-bound intermediates were assigned for each reaction step by HPLC-MS. Native mass spectrometry experiments identified four stable complexes: the acyl-carrier proteins ApeE and ApeF bound to the thioesterase ApeK, the dehydratases ApeI and ApeP, and the ketosynthase ApeO in complex with its chain-length factor ApeC. X-ray structures of the heterodimeric ApeO:ApeC and ApeI:ApeP complexes depict striking protein-protein interactions. Altogether, our study elucidated mechanistic aspects of APE biosynthesis that unifies elements of type II fatty acid and PKS systems, but in addition includes novel enzyme complexes.
Organizational Affiliation:
Molekulare Biotechnologie, Fachbereich Biowissenschaften, Goethe-Universität Frankfurt am Main and Buchmann Institute for Molecular Life Sciences (BMLS) , Goethe-Universität Frankfurt , Max-von-Laue-Straße 9 and 15 , 60438 Frankfurt am Main , Germany.