An Uncommon Type II PKS Catalyzes Biosynthesis of Aryl Polyene Pigments.
Grammbitter, G.L.C., Schmalhofer, M., Karimi, K., Shi, Y.M., Schoner, T.A., Tobias, N.J., Morgner, N., Groll, M., Bode, H.B.(2019) J Am Chem Soc 141: 16615-16623
- PubMed: 30908039
- DOI: https://doi.org/10.1021/jacs.8b10776
- Primary Citation of Related Structures:
6QSP, 6QSR - PubMed Abstract:
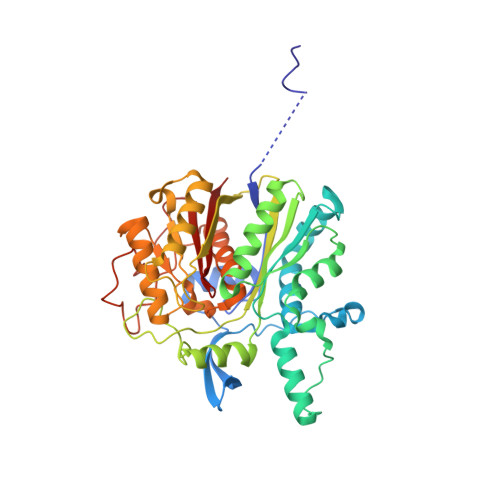
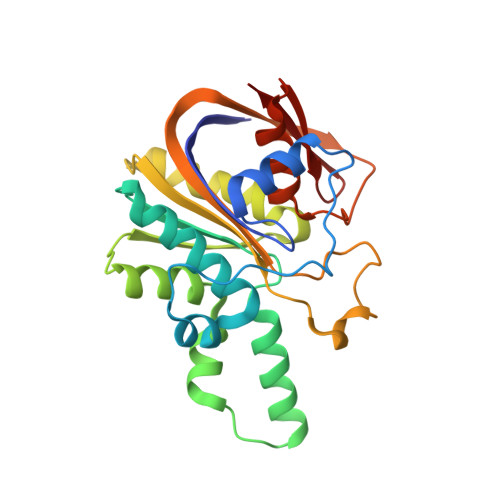
Aryl polyene (APE) pigments are a widely distributed class of bacterial polyketides. So far, little is known about the biosynthesis of these compounds, which are produced by a novel type II polyketide synthase (PKS). We have identified all enzymes involved in APE biosynthesis and determined their peculiar functions. The biosynthesis was reconstituted in vitro , and ACP-bound intermediates were assigned for each reaction step by HPLC-MS. Native mass spectrometry experiments identified four stable complexes: the acyl-carrier proteins ApeE and ApeF bound to the thioesterase ApeK, the dehydratases ApeI and ApeP, and the ketosynthase ApeO in complex with its chain-length factor ApeC. X-ray structures of the heterodimeric ApeO:ApeC and ApeI:ApeP complexes depict striking protein-protein interactions. Altogether, our study elucidated mechanistic aspects of APE biosynthesis that unifies elements of type II fatty acid and PKS systems, but in addition includes novel enzyme complexes.
- Molekulare Biotechnologie, Fachbereich Biowissenschaften, Goethe-Universität Frankfurt am Main and Buchmann Institute for Molecular Life Sciences (BMLS) , Goethe-Universität Frankfurt , Max-von-Laue-Straße 9 and 15 , 60438 Frankfurt am Main , Germany.
Organizational Affiliation: