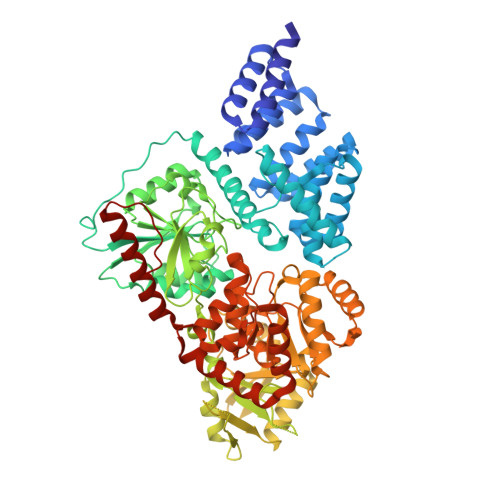
Structure-Based Evolution of Low Nanomolar O-GlcNAc Transferase Inhibitors.
Martin, S.E.S., Tan, Z.W., Itkonen, H.M., Duveau, D.Y., Paulo, J.A., Janetzko, J., Boutz, P.L., Tork, L., Moss, F.A., Thomas, C.J., Gygi, S.P., Lazarus, M.B., Walker, S.(2018) J Am Chem Soc 140: 13542-13545
- PubMed: 30285435
- DOI: https://doi.org/10.1021/jacs.8b07328
- Primary Citation of Related Structures:
6MA1, 6MA2, 6MA3, 6MA4, 6MA5 - PubMed Abstract:
Reversible glycosylation of nuclear and cytoplasmic proteins is an important regulatory mechanism across metazoans. One enzyme, O-linked N-acetylglucosamine transferase (OGT), is responsible for all nucleocytoplasmic glycosylation and there is a well-known need for potent, cell-permeable inhibitors to interrogate OGT function. Here we report the structure-based evolution of OGT inhibitors culminating in compounds with low nanomolar inhibitory potency and on-target cellular activity. In addition to disclosing useful OGT inhibitors, the structures we report provide insight into how to inhibit glycosyltransferases, a family of enzymes that has been notoriously refractory to inhibitor development.
Organizational Affiliation:
Department of Microbiology , Harvard Medical School , Boston , Massachusetts 02115 , United States.