RanL182A in complex with RanBP1-CRM1
Sun, Q., Zhang, Y.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| GTP-binding nuclear protein Ran | 216 | Homo sapiens | Mutation(s): 1 Gene Names: RAN, ARA24, OK/SW-cl.81 | 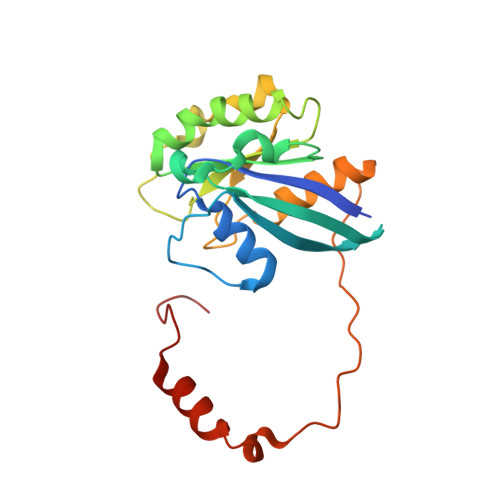 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P62826 (Homo sapiens) Explore P62826 Go to UniProtKB: P62826 | |||||
PHAROS: P62826 GTEx: ENSG00000132341 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P62826 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ran-specific GTPase-activating protein 1 | 140 | Saccharomyces cerevisiae S288C | Mutation(s): 0 Gene Names: YRB1, CST20, HTN1, SFO1, YDR002W, YD8119.08 | 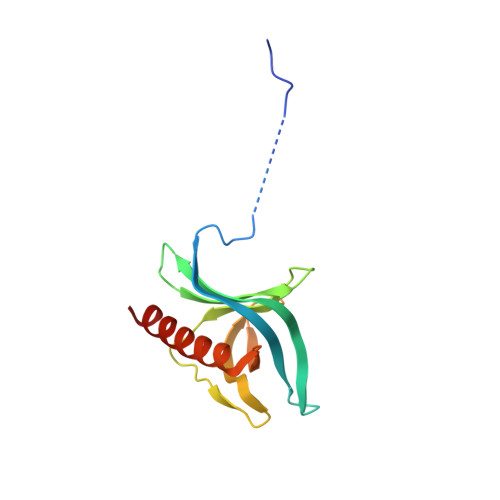 | |
UniProt | |||||
Find proteins for P41920 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore P41920 Go to UniProtKB: P41920 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P41920 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Exportin-1,Exportin-1 | 1,024 | Saccharomyces cerevisiae S288C | Mutation(s): 5 Gene Names: CRM1, KAP124, XPO1, YGR218W, G8514 | 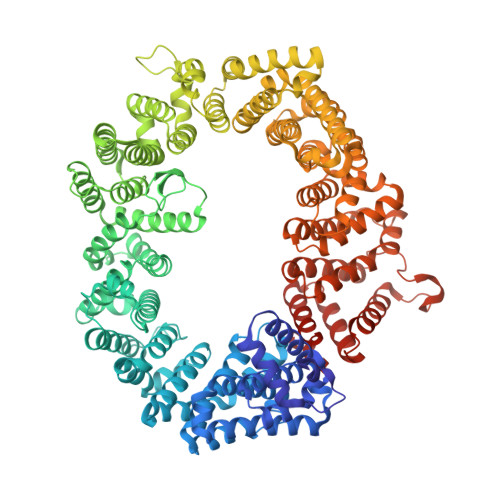 | |
UniProt | |||||
Find proteins for P30822 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore P30822 Go to UniProtKB: P30822 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P30822 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 6 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| GTP Query on GTP | D [auth A] | GUANOSINE-5'-TRIPHOSPHATE C10 H16 N5 O14 P3 XKMLYUALXHKNFT-UUOKFMHZSA-N |  | ||
| F2X (Subject of Investigation/LOI) Query on F2X | I [auth C], J [auth C] | (2~{R})-2-methyl-5-oxidanyl-2,3-dihydronaphthalene-1,4-dione C11 H10 O3 ALPCEXCHMFUSAN-ZCFIWIBFSA-N |  | ||
| NO3 Query on NO3 | G [auth A], H [auth A], M [auth C], N [auth C] | NITRATE ION N O3 NHNBFGGVMKEFGY-UHFFFAOYSA-N |  | ||
| CL Query on CL | F [auth A], K [auth C] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| MG Query on MG | E [auth A] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| NA Query on NA | L [auth C] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 105.063 | α = 90 |
| b = 105.063 | β = 90 |
| c = 306.534 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| HKL-2000 | data scaling |
| PDB_EXTRACT | data extraction |
| HKL-2000 | data reduction |
| MOLREP | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | 81502629 |