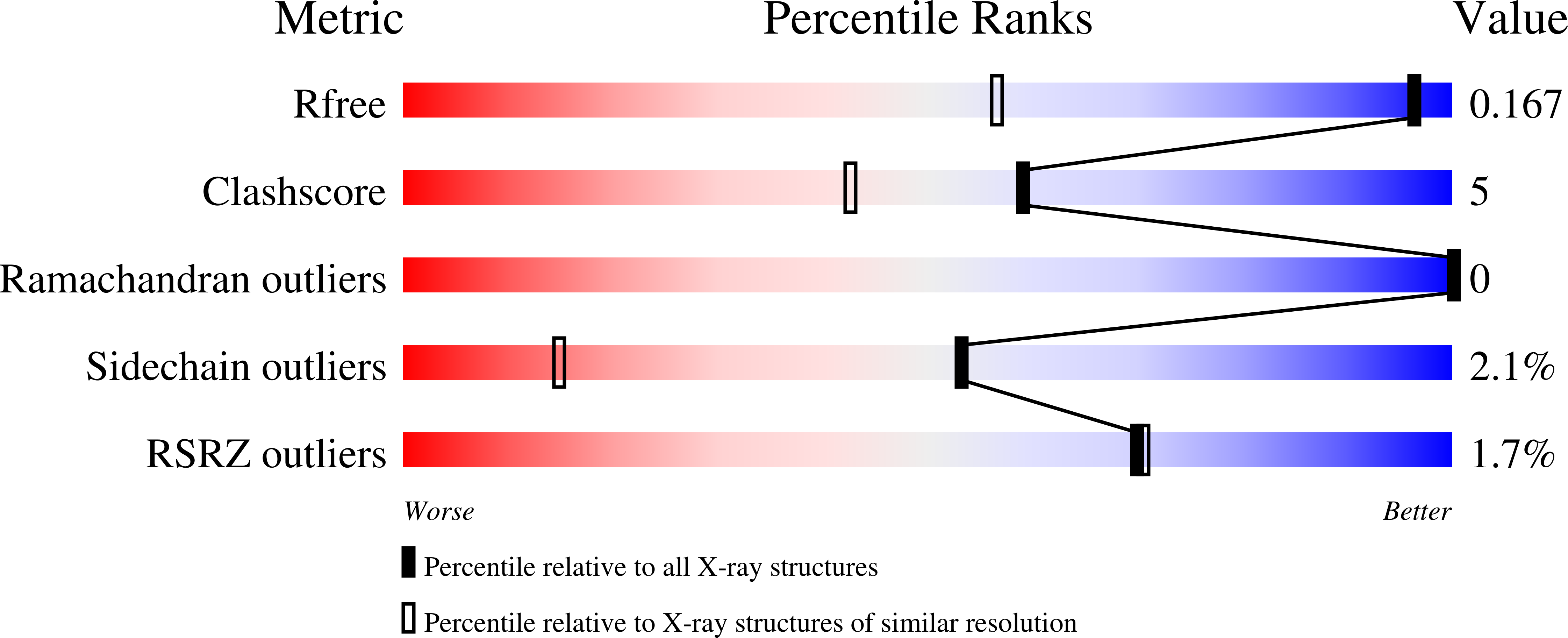
Electrochemical and structural characterization of Azotobacter vinelandii flavodoxin II.
Segal, H.M., Spatzal, T., Hill, M.G., Udit, A.K., Rees, D.C.(2017) Protein Sci 26: 1984-1993
- PubMed: 28710816
- DOI: https://doi.org/10.1002/pro.3236
- Primary Citation of Related Structures:
5K9B - PubMed Abstract:
Azotobacter vinelandii flavodoxin II serves as a physiological reductant of nitrogenase, the enzyme system mediating biological nitrogen fixation. Wildtype A. vinelandii flavodoxin II was electrochemically and crystallographically characterized to better understand the molecular basis for this functional role. The redox properties were monitored on surfactant-modified basal plane graphite electrodes, with two distinct redox couples measured by cyclic voltammetry corresponding to reduction potentials of -483 ± 1 mV and -187 ± 9 mV (vs. NHE) in 50 mM potassium phosphate, 150 mM NaCl, pH 7.5. These redox potentials were assigned as the semiquinone/hydroquinone couple and the quinone/semiquinone couple, respectively. This study constitutes one of the first applications of surfactant-modified basal plane graphite electrodes to characterize the redox properties of a flavodoxin, thus providing a novel electrochemical method to study this class of protein. The X-ray crystal structure of the flavodoxin purified from A. vinelandii was solved at 1.17 Å resolution. With this structure, the native nitrogenase electron transfer proteins have all been structurally characterized. Docking studies indicate that a common binding site surrounding the Fe-protein [4Fe:4S] cluster mediates complex formation with the redox partners Mo-Fe protein, ferredoxin I, and flavodoxin II. This model supports a mechanistic hypothesis that electron transfer reactions between the Fe-protein and its redox partners are mutually exclusive.
Organizational Affiliation:
Division of Chemistry and Chemical Engineering, Howard Hughes Medical Institute, California Institute of Technology, Pasadena, California, 91125.