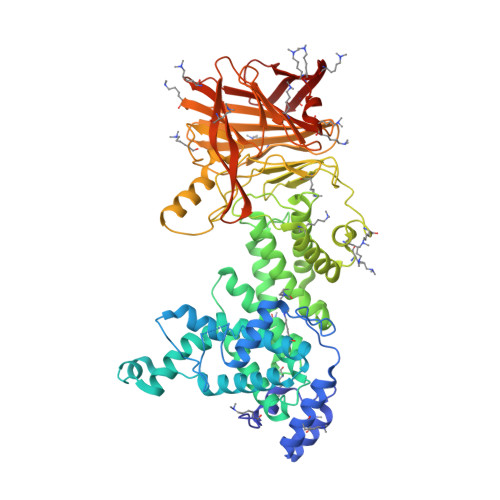
Conformational flexibility of PL12 family heparinases: structure and substrate specificity of heparinase III from Bacteroides thetaiotaomicron (BT4657).
Ulaganathan, T., Shi, R., Yao, D., Gu, R.X., Garron, M.L., Cherney, M., Tieleman, D.P., Sterner, E., Li, G., Li, L., Linhardt, R.J., Cygler, M.(2017) Glycobiology 27: 176-187
- PubMed: 27621378
- DOI: https://doi.org/10.1093/glycob/cww096
- Primary Citation of Related Structures:
5JMD, 5JMF - PubMed Abstract:
Glycosaminoglycans (GAGs) are linear polysaccharides comprised of disaccharide repeat units, a hexuronic acid, glucuronic acid or iduronic acid, linked to a hexosamine, N-acetylglucosamine (GlcNAc) or N-acetylgalactosamine. GAGs undergo further modification such as epimerization and sulfation. These polysaccharides are abundant in the extracellular matrix and connective tissues. GAGs function in stabilization of the fibrillar extracellular matrix, control of hydration, regulation of tissue, organism development by controlling cell cycle, cell behavior and differentiation. Niche adapted bacteria express enzymes called polysaccharide lyases (PL), which degrade GAGs for their nutrient content. PL have been classified into 24 sequence-related families. Comparison of 3D structures of the prototypic members of these families allowed identification of distant evolutionary relationships between lyases that were unrecognized at the sequence level, and identified occurrences of convergent evolution. We have characterized structurally and enzymatically heparinase III from Bacteroides thetaiotaomicron (BtHepIII; gene BT4657), which is classified within the PL12 family. BtHepIII is a 72.5 kDa protein. We present the X-ray structures of two crystal forms of BtHepIII at resolution 1.8 and 2.4 Å. BtHepIII contains two domains, the N-terminal α-helical domain forming a toroid and the C-terminal β-sheet domain. Comparison with recently determined structures of two other heparinases from the same PL12 family allowed us to identify structural flexibility in the arrangement of the domains indicating open-close movement. Based on comparison with other GAG lyases, we identified Tyr301 as the main catalytic residue and confirmed this by site-directed mutagenesis. We have characterized substrate preference of BtHepIII toward sulfate-poor heparan sulfate substrate.
Organizational Affiliation:
Department of Biochemistry, University of Saskatchewan, Saskatoon, S7N 5E5 Saskatchewan, Canada.