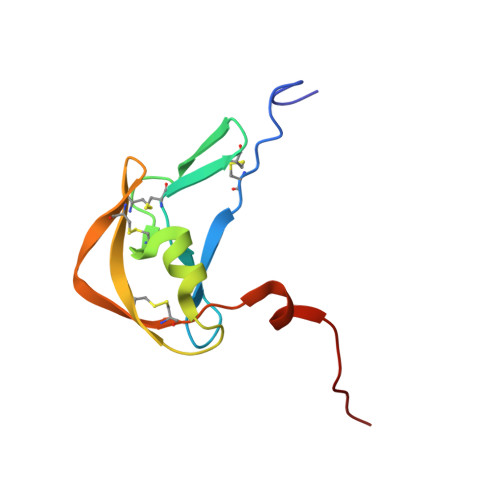
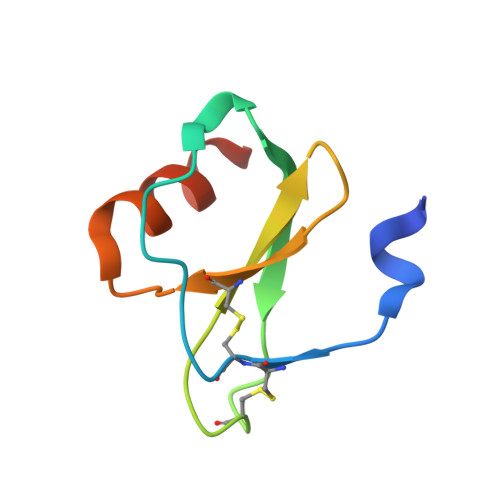
Structural basis of chemokine sequestration by a tick chemokine binding protein: the crystal structure of the complex between Evasin-1 and CCL3
Dias, J.M., Losberger, C., Deruaz, M., Power, C.A., Proudfoot, A.E.I., Shaw, J.P.(2009) PLoS One 4
- PubMed: 20041127
- DOI: https://doi.org/10.1371/journal.pone.0008514
- Primary Citation of Related Structures:
3FPR, 3FPT, 3FPU - PubMed Abstract:
Chemokines are a subset of cytokines responsible for controlling the cellular migration of inflammatory cells through interaction with seven transmembrane G protein-coupled receptors. The blocking of a chemokine-receptor interaction results in a reduced inflammatory response, and represents a possible anti-inflammatory strategy, a strategy that is already employed by some virus and parasites. Anti-chemokine activity has been described in the extracts of tick salivary glands, and we have recently described the cloning and characterization of such chemokine binding proteins from the salivary glands, which we have named Evasins.
Organizational Affiliation:
Merck Serono Geneva Research Center, Merck Serono S.A., Geneva, Switzerland.