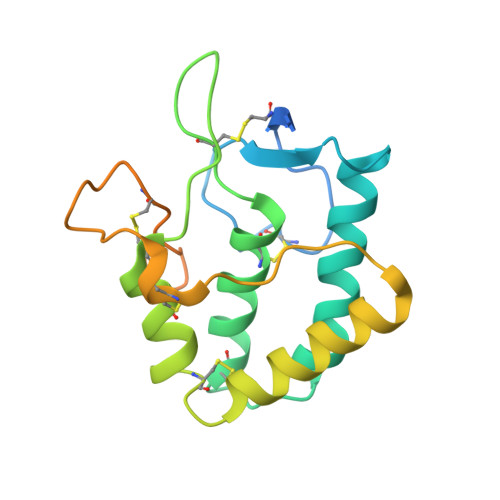
Crystal structure of the frizzled-like cysteine-rich domain of the receptor tyrosine kinase MuSK.
Stiegler, A.L., Burden, S.J., Hubbard, S.R.(2009) J Mol Biol 393: 1-9
- PubMed: 19664639
- DOI: https://doi.org/10.1016/j.jmb.2009.07.091
- Primary Citation of Related Structures:
3HKL - PubMed Abstract:
Muscle-specific kinase (MuSK) is an essential receptor tyrosine kinase for the establishment and maintenance of the neuromuscular junction (NMJ). Activation of MuSK by agrin, a neuronally derived heparan-sulfate proteoglycan, and LRP4 (low-density lipoprotein receptor-related protein-4), the agrin receptor, leads to clustering of acetylcholine receptors on the postsynaptic side of the NMJ. The ectodomain of MuSK comprises three immunoglobulin-like domains and a cysteine-rich domain (Fz-CRD) related to those in Frizzled proteins, the receptors for Wnts. Here, we report the crystal structure of the MuSK Fz-CRD at 2.1 A resolution. The structure reveals a five-disulfide-bridged domain similar to CRDs of Frizzled proteins but with a divergent C-terminal region. An asymmetric dimer present in the crystal structure implicates surface hydrophobic residues that may function in homotypic or heterotypic interactions to mediate co-clustering of MuSK, rapsyn, and acetylcholine receptors at the NMJ.
Organizational Affiliation:
Structural Biology Program, Kimmel Center for Biology and Medicine of the Skirball Institute, New York University School of Medicine, New York, NY 10016, USA.