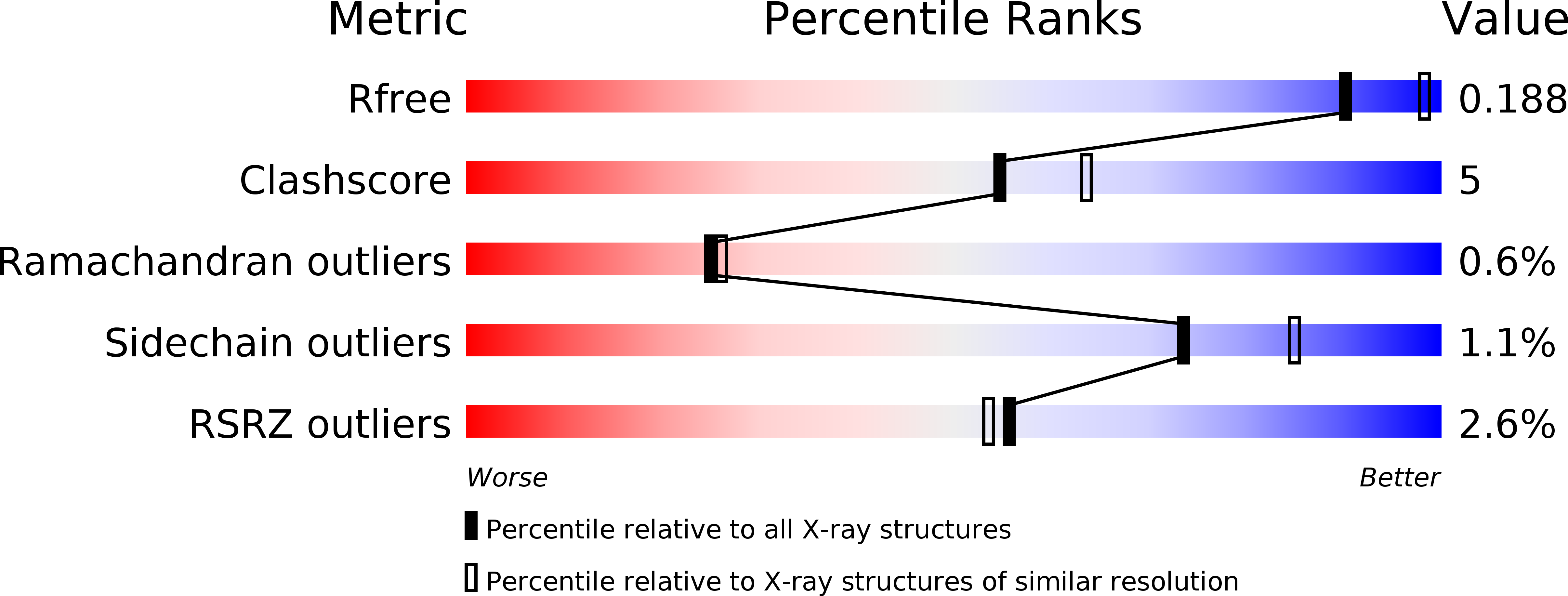
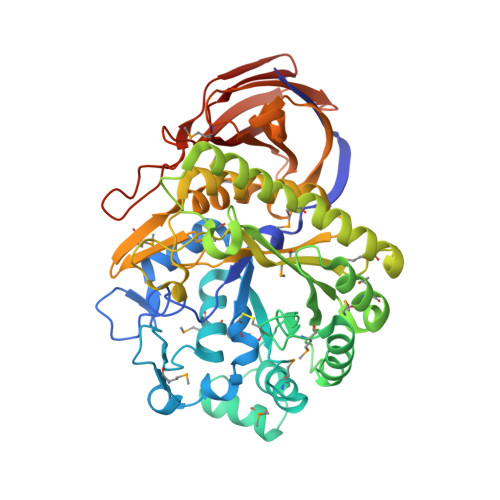
The Structure of the Complex between a Branched Pentasaccharide and Thermobacillus Xylanilyticus Gh-51 Arabinofuranosidase Reveals Xylan-Binding Determinants and Induced Fit.
Paes, G., Skov, L.K., O'Donohue, M.J., Remond, C., Kastrup, J.S., Gajhede, M., Mirza, O.(2008) Biochemistry 47: 7441
- PubMed: 18563919
- DOI: https://doi.org/10.1021/bi800424e
- Primary Citation of Related Structures:
2VRK, 2VRQ - PubMed Abstract:
The crystal structure of the family GH-51 alpha- l-arabinofuranosidase from Thermobacillus xylanilyticus has been solved as a seleno-methionyl derivative. In addition, the structure of an inactive mutant Glu176Gln is presented in complex with a branched pentasaccharide, a fragment of its natural substrate xylan. The overall structure shows the two characteristic GH-51 domains: a catalytic domain that is folded into a (beta/alpha) 8-barrel and a C-terminal domain that displays jelly roll architecture. The pentasaccharide is bound in a groove on the surface of the enzyme, with the mono arabinosyl branch entering a tight pocket harboring the catalytic dyad. Detailed analyses of both structures and comparisons with the two previously determined structures from Geobacillus stearothermophilus and Clostridium thermocellum reveal important details unique to the Thermobacillus xylanilyticus enzyme. In the absence of substrate, the enzyme adopts an open conformation. In the substrate-bound form, the long loop connecting beta-strand 2 to alpha-helix 2 closes the active site and interacts with the substrate through residues His98 and Trp99. The results of kinetic and fluorescence titration studies using mutants underline the importance of this loop, and support the notion of an interaction between Trp99 and the bound substrate. We suggest that the changes in loop conformation are an integral part of the T. xylanilyticus alpha- l-arabinofuranosidase reaction mechanism, and ensure efficient binding and release of substrate.
Organizational Affiliation:
INRAUMR FARE 614, 8, rue Gabriel Voisin, BP 316, 51688 Reims cedex 2, France.