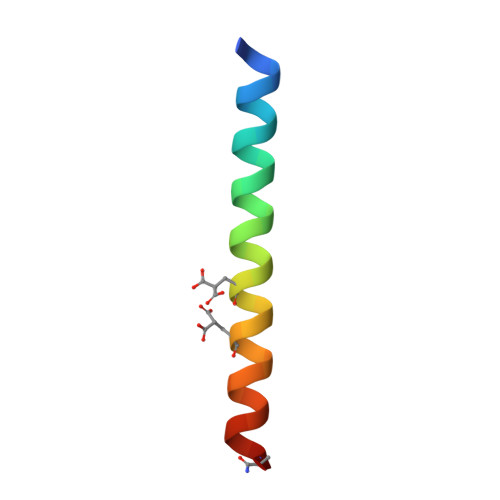
Structure of a designed, right-handed coiled-coil tetramer containing all biological amino acids.
Sales, M., Plecs, J.J., Holton, J.M., Alber, T.(2007) Protein Sci 16: 2224-2232
- PubMed: 17766380
- DOI: https://doi.org/10.1110/ps.062702907
- Primary Citation of Related Structures:
2O6N - PubMed Abstract:
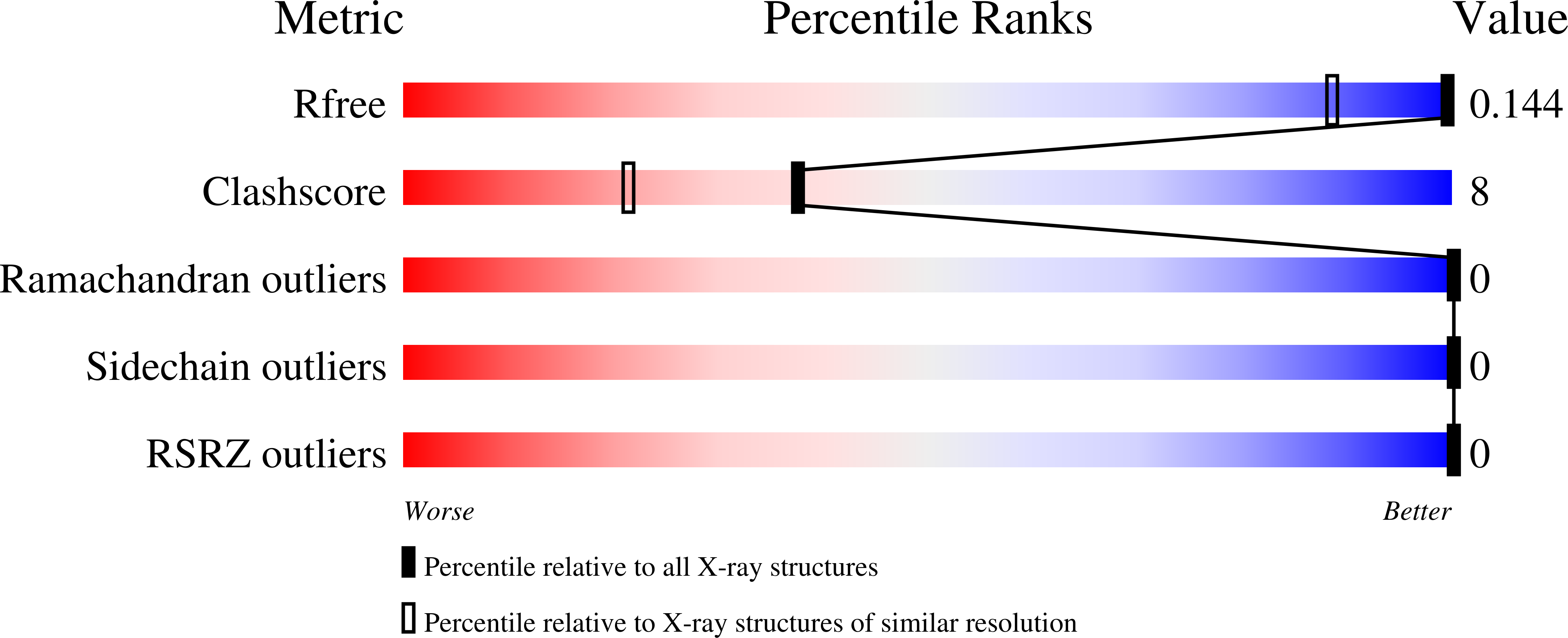
The previous design of an unprecedented family of two-, three-, and four-helical, right-handed coiled coils utilized nonbiological amino acids to efficiently pack spaces in the oligomer cores. Here we show that a stable, right-handed parallel tetrameric coiled coil, called RH4B, can be designed entirely using biological amino acids. The X-ray crystal structure of RH4B was determined to 1.1 Angstrom resolution using a designed metal binding site to coordinate a single Yb(2+) ion per 33-amino acid polypeptide chain. The resulting experimental phases were particularly accurate, and the experimental electron density map provided an especially clear, unbiased view of the molecule. The RH4B structure closely matched the design, with equivalent core rotamers and an overall root-mean-square deviation for the N-terminal repeat of the tetramer of 0.24 Angstrom. The clarity and resolution of the electron density map, however, revealed alternate rotamers and structural differences between the three sequence repeats in the molecule. These results suggest that the RH4B structure populates an unanticipated variety of structures.
Organizational Affiliation:
Department of Physics, University of California, Berkeley, California 94720-3206, USA.