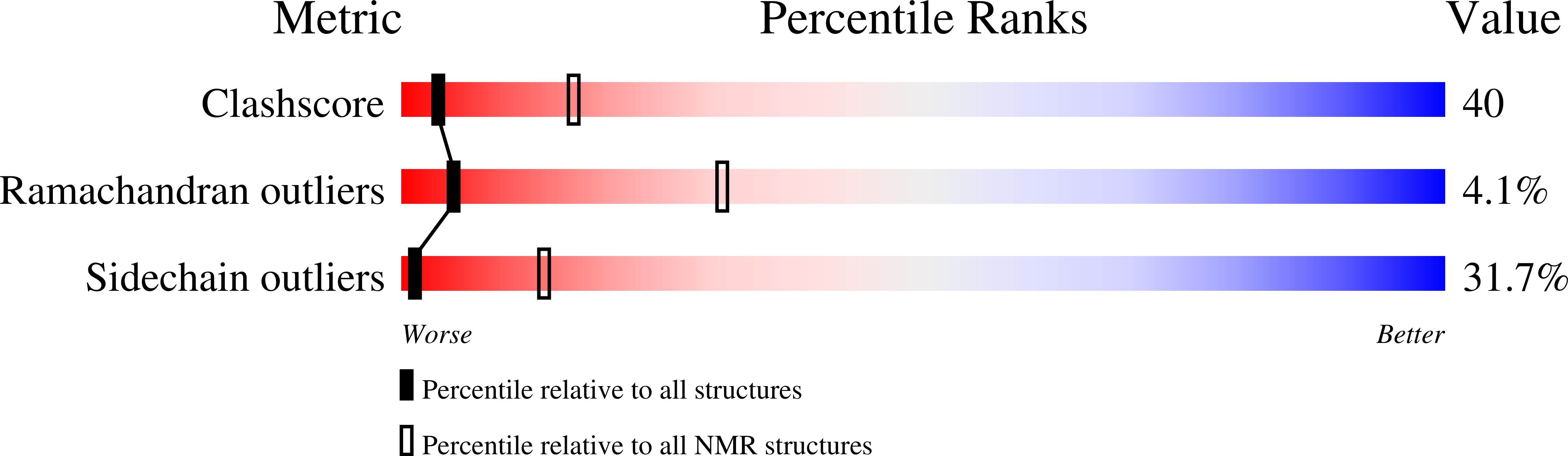The solution structure and dynamics of human pancreatic ribonuclease determined by NMR spectroscopy provide insight into its remarkable biological activities and inhibition.
Kover, K.E., Bruix, M., Santoro, J., Batta, G., Laurents, D.V., Rico, M.(2008) J Mol Biol 379: 953-965
- PubMed: 18495155
- DOI: https://doi.org/10.1016/j.jmb.2008.04.042
- Primary Citation of Related Structures:
2K11 - PubMed Abstract:
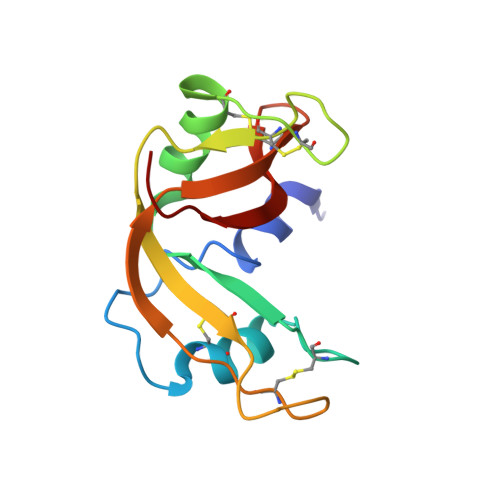
Human pancreatic ribonuclease (RNase 1) is expressed in many tissues; has several important enzymatic and biological activities, including efficient cleavage of single-stranded RNA, double-stranded RNA and double-stranded RNA-DNA hybrids, digestion of dietary RNA, regulation of vascular homeostasis, inactivation of the HIV, activation of immature dendritic cells and induction of cytokine production; and furthermore shows potential as an anti-tumor agent. The solution structure and dynamics of uncomplexed, wild-type RNase 1 have been determined by NMR spectroscopy methods to better understand these activities. The family of 20 structures determined on the basis of 6115 unambiguous nuclear Overhauser enhancements is well resolved (pairwise backbone RMSD=1.07 A) and has the classic RNase A type of tertiary structure. Important structural differences compared with previously determined crystal structures of RNase 1 variants or inhibitor-bound complexes are observed in the conformation of loop regions and side chains implicated in the enzymatic as well as biological activities and binding to the cytoplasmic RNase inhibitor. Multiple side chain conformations observed for key surface residues are proposed to be crucial for membrane binding as well as translocation and efficient RNA hydrolysis. (15)N-(1)H relaxation measurements interpreted with the standard and our extended Lipari-Szabo formalism reveal rigid regions and identify more dynamic loop regions. Some of the most dynamic areas are key for binding to the cytoplasmic RNase inhibitor. This finding and the important differences observed between the structure in solution and that bound to the inhibitor are indications that RNase 1 to inhibitor binding can be better described by the "induced fit" model rather than the rigid "lock-into-key" mechanism. Translational diffusion measurements reveal that RNase 1 is predominantly dimeric above 1 mM concentration; the possible implications of this dimeric state for the remarkable biological properties of RNase 1 are discussed.
Organizational Affiliation:
Department of Chemistry, University of Debrecen, 4010 Debrecen, Hungary.