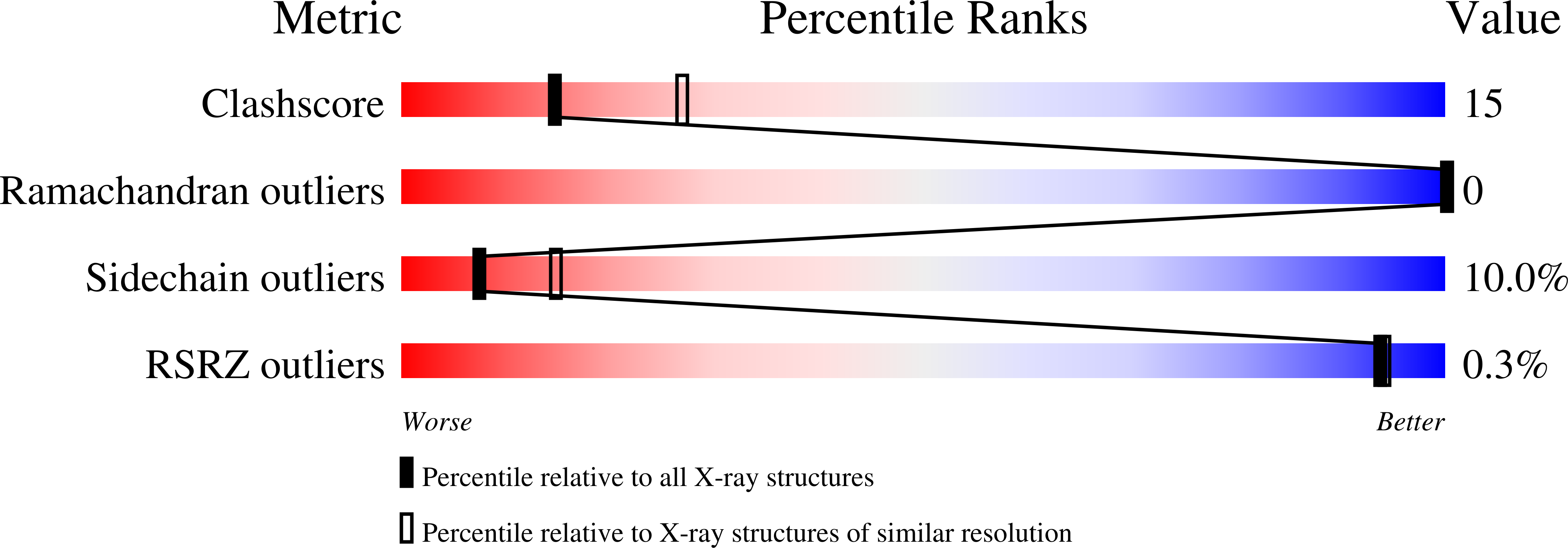Buried charged surface in proteins.
Kajander, T., Kahn, P.C., Passila, S.H., Cohen, D.C., Lehtio, L., Adolfsen, W., Warwicker, J., Schell, U., Goldman, A.(2000) Structure 8: 1203-1214
- PubMed: 11080642
- DOI: https://doi.org/10.1016/s0969-2126(00)00520-7
- Primary Citation of Related Structures:
1F9C - PubMed Abstract:
The traditional picture of charged amino acids in globular proteins is that they are almost exclusively on the outside exposed to the solvent. Buried charges, when they do occur, are assumed to play an essential role in catalysis and ligand binding, or in stabilizing structure as, for instance, helix caps.
Organizational Affiliation:
Centre for Biotechnology, University of Turku, Turku, Finland.