Characterization of the zinc finger mu-protein HVO_0758 from Haloferax volcanii : biological roles, zinc binding, and NMR solution structure.
Uresin, D., Pyper, D.J., Borst, A., Hadjeras, L., Gelhausen, R., Backofen, R., Sharma, C., Schwalbe, H., Soppa, J.(2023) Front Microbiol 14: 1280972-1280972
- PubMed: 38094630
- DOI: https://doi.org/10.3389/fmicb.2023.1280972
- Primary Citation of Related Structures:
8Q5B - PubMed Abstract:
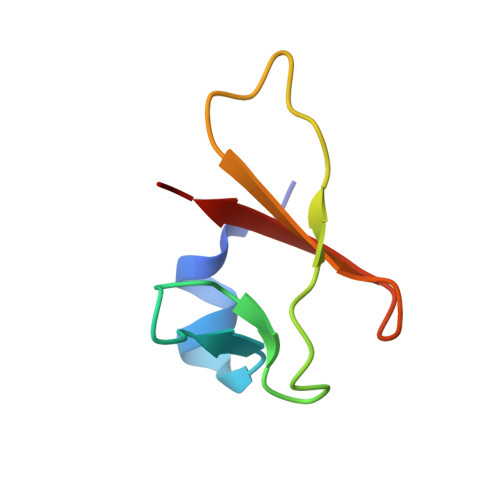
It is increasingly recognized that very small proteins (μ-proteins) are ubiquitously found in all species of the three domains of life, and that they fulfill important functions. The halophilic archaeon Haloferax volcanii contains 282 μ-proteins of less than 70 amino acids. Notably, 43 of these contain two C(P)XCG motifs, suggesting their potential to complex a zinc ion. To explore the significance of these proteins, 16 genes encoding C(P)XCG proteins had been deleted, and the majority of mutants exhibited phenotypic differences to the wild-type. One such protein, HVO_2753, was thoroughly characterized in a previous study. In the present study an in-depth analysis of a second protein, HVO_0758, was performed. To achieve this goal, the HVO_0758 protein was produced heterologously in Escherichia coli and homologously in H. volcanii . The purified protein was characterized using various biochemical approaches and NMR spectroscopy. The findings demonstrated that HVO_0758 is indeed a bona fide zinc finger protein, and that all four cysteine residues are essential for folding. The NMR solution structure was solved, revealing that HVO_0758 is comprised of an N-terminal alpha helix containing several positively charged residues and a globular core with the zinc finger domain. The transcriptomes of the HVO_0758 deletion mutant and, for comparison, the HVO_2753 deletion mutant were analyzed with RNA-Seq and compared against that of the wild-type. In both mutants many motility and chemotaxis genes were down-regulated, in agreement to the phenotype of the deletion mutants, which had a swarming deficit. The two H. volcanii zinc-finger μ-proteins HVO_0758 and HVO_2753 showed many differences. Taken together, two zinc finger μ-proteins of H. volcanii have been characterized intensively, which emerged as pivotal contributors to swarming behavior and biofilm formation.
Organizational Affiliation:
Institute for Molecular Biosciences, Goethe University, Frankfurt, Germany.