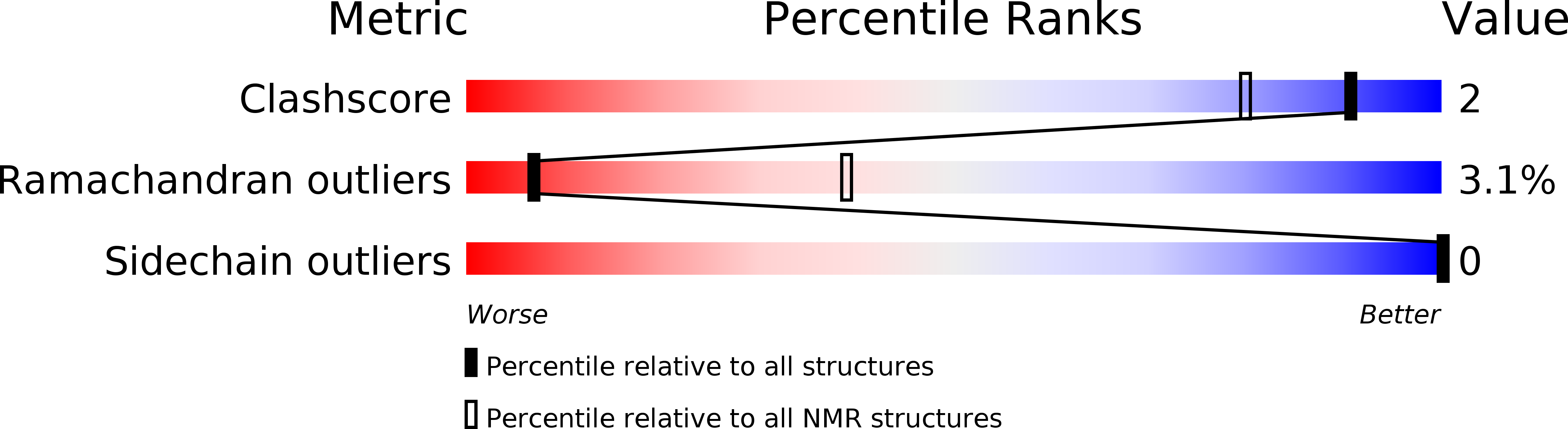Membrane interactions of the anuran antimicrobial peptide HSP1-NH2: Different aspects of the association to anionic and zwitterionic biomimetic systems.
Gomes, I.P., Santos, T.L., de Souza, A.N., Nunes, L.O., Cardoso, G.A., Matos, C.O., Costa, L.M.F., Liao, L.M., Resende, J.M., Verly, R.M.(2020) Biochim Biophys Acta Biomembr 1863: 183449-183449
- PubMed: 32828849
- DOI: https://doi.org/10.1016/j.bbamem.2020.183449
- Primary Citation of Related Structures:
6WPB, 6WPD - PubMed Abstract:
Studies have suggested that antimicrobial peptides act by different mechanisms, such as micellisation, self-assembly of nanostructures and pore formation on the membrane surface. This work presents an extensive investigation of the membrane interactions of the 14 amino-acid antimicrobial peptide hylaseptin P1-NH 2 (HSP1-NH 2 ), derived from the tree-frog Hyla punctata, which has stronger antifungal than antibacterial potential. Biophysical and structural analyses were performed and the correlated results were used to describe in detail the interactions of HSP1-NH 2 with zwitterionic and anionic detergent micelles and phospholipid vesicles. HSP1-NH 2 presents similar well-defined helical conformations in both zwitterionic and anionic micelles, although NMR spectroscopy revealed important structural differences in the peptide N-terminus. 2 H exchange experiments of HSP1-NH 2 indicated the insertion of the most N-terminal residues (1-3) in the DPC-d 38 micelles. A higher enthalpic contribution was verified for the interaction of the peptide with anionic vesicles in comparison with zwitterionic vesicles. The pore formation ability of HSP1-NH 2 (examined by dye release assays) and its effect on the size and surface charge as well as on the lipid acyl chain ordering (evaluated by Fourier-transform infrared spectroscopy) of anionic phospholipid vesicles showed membrane disruption even at low peptide-to-phospholipid ratios, and the effect increases proportionately to the peptide concentration. On the other hand, these biophysical investigations showed that a critical peptide-to-phospholipid ratio around 0.6 is essential for promoting disruption of zwitterionic membranes. In conclusion, this study demonstrates that the binding process of the antimicrobial HSP1-NH 2 peptide depends on the membrane composition and peptide concentration.
Organizational Affiliation:
Departamento de Química - Universidade Federal dos Vales do Jequitinhonha e Mucuri, 39100-000 Diamantina, MG, Brazil.