19F NMR Monitoring of Reversible Protein Post-Translational Modifications: Class D beta-Lactamase Carbamylation and Inhibition.
van Groesen, E., Lohans, C.T., Brem, J., Aertker, K.M.J., Claridge, T.D.W., Schofield, C.J.(2019) Chemistry 25: 11837-11841
- PubMed: 31310409
- DOI: https://doi.org/10.1002/chem.201902529
- Primary Citation of Related Structures:
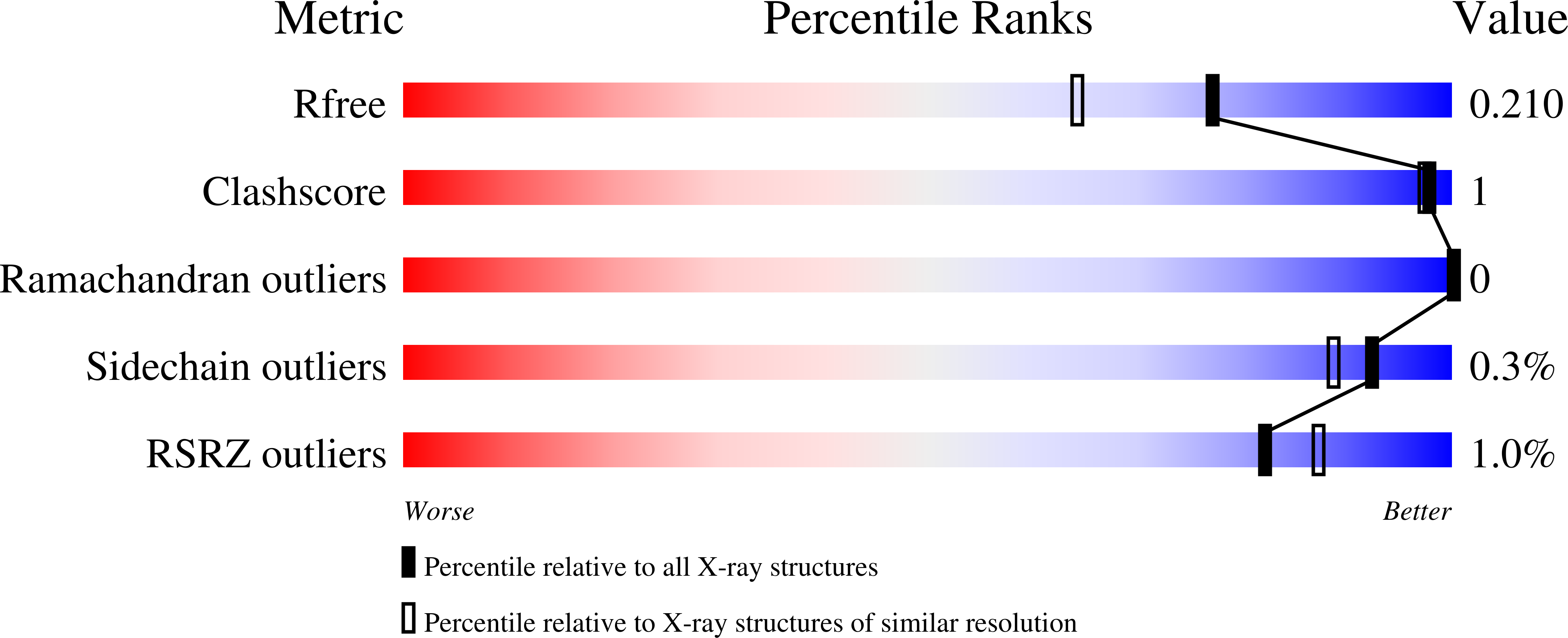
6RJ7 - PubMed Abstract:
Bacterial production of β-lactamases with carbapenemase activity is a global health threat. The active sites of class D carbapenemases such as OXA-48, which is of major clinical importance, uniquely contain a carbamylated lysine residue which is essential for catalysis. Although there is significant interest in characterizing this post-translational modification, and it is a promising inhibition target, protein carbamylation is challenging to monitor in solution. We report the use of 19 F NMR spectroscopy to monitor the carbamylation state of 19 F-labelled OXA-48. This method was used to investigate the interactions of OXA-48 with clinically used serine β-lactamase inhibitors, including avibactam and vaborbactam. Crystallographic studies on 19 F-labelled OXA-48 provide a structural rationale for the sensitivity of the 19 F label to active site interactions. The overall results demonstrate the use of 19 F NMR to monitor reversible covalent post-translational modifications.
Organizational Affiliation:
Department of Chemistry, University of Oxford, Oxford, OX1 3TA, UK.