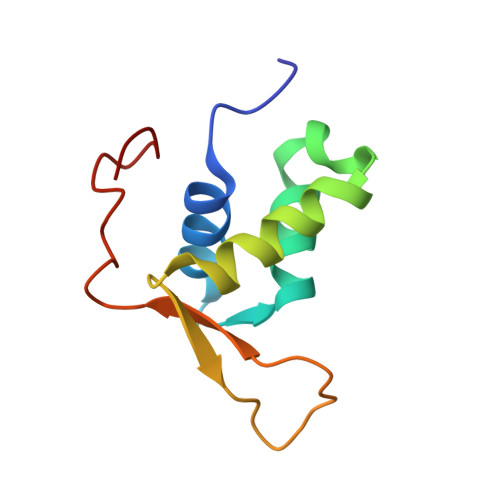
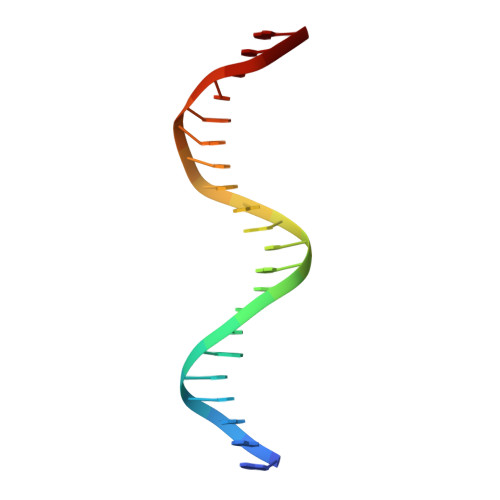
Crystal Structure of FOXC2 in Complex with DNA Target.
Li, S., Pradhan, L., Ashur, S., Joshi, A., Nam, H.J.(2019) ACS Omega 4: 10906-10914
- PubMed: 31460188
- DOI: https://doi.org/10.1021/acsomega.9b00756
- Primary Citation of Related Structures:
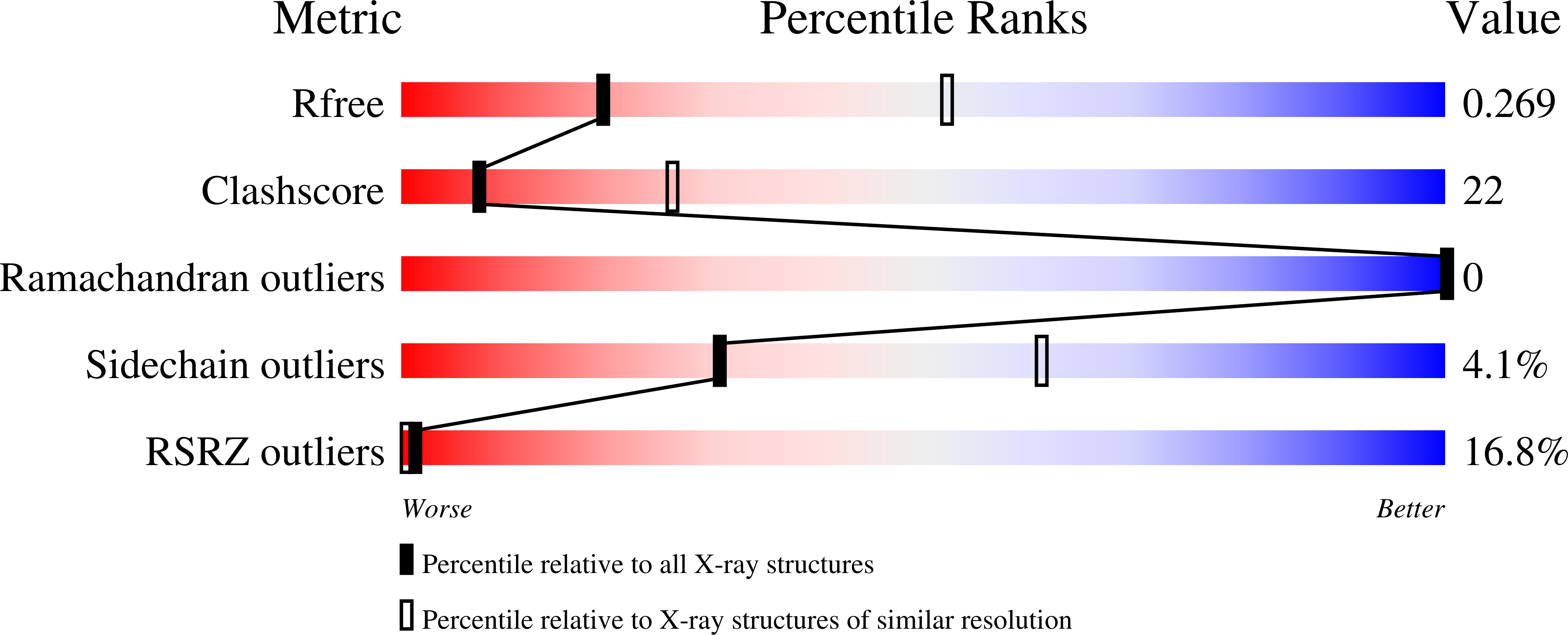
6O3T - PubMed Abstract:
Forkhead transcription factor C2 (FOXC2) is a transcription factor regulating vascular and lymphatic development, and its mutations are linked to lymphedema-distichiasis syndrome. FOXC2 is also a crucial regulator of the epithelial-mesenchymal transition processes essential for tumor metastasis. Here, we report the crystal structure of the FOXC2-DNA-binding domain in complex with its cognate DNA. The crystal structure provides the basis of DNA sequence recognition by FOXC2 for the T/CAAAC motif. Helix 3 makes the majority of the DNA-protein interactions and confers the DNA sequence specificity. The computational energy calculation results also validate the structural observations. The FOXC2 and DNA complex structure provides a detailed picture of protein and DNA interactions, which allows us to predict its DNA recognition specificity and impaired functions in mutants identified in human patients.
Organizational Affiliation:
Department of Bioengineering, The University of Texas at Dallas, 800 W. Campbell Road, Richardson, Texas 75080, United States.