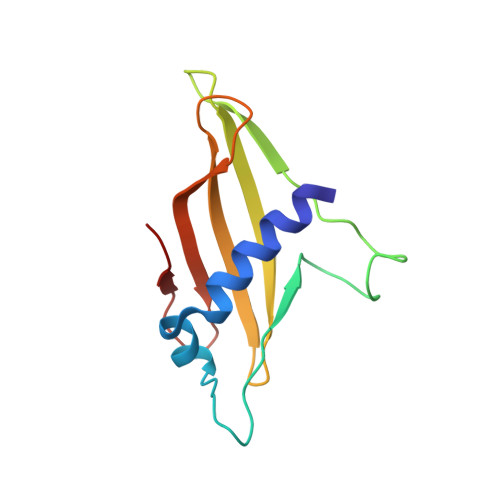
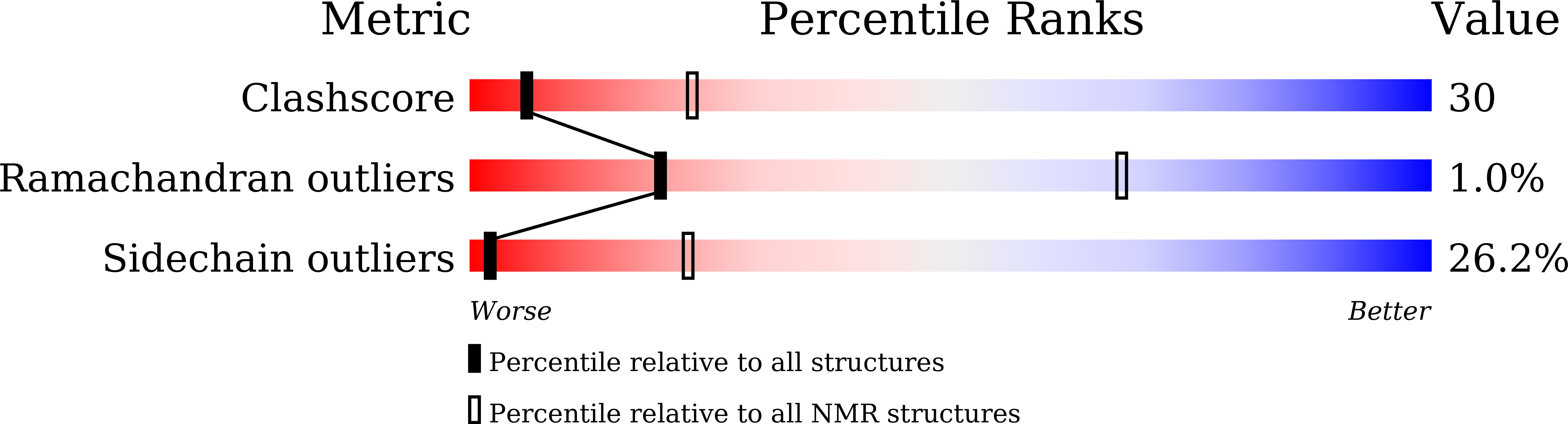Crystal contact-free conformation of an intrinsically flexible loop in protein crystal: Tim21 as the case study.
Bala, S., Shinya, S., Srivastava, A., Ishikawa, M., Shimada, A., Kobayashi, N., Kojima, C., Tama, F., Miyashita, O., Kohda, D.(2020) Biochim Biophys Acta Gen Subj 1864: 129418-129418
- PubMed: 31449839
- DOI: https://doi.org/10.1016/j.bbagen.2019.129418
- Primary Citation of Related Structures:
6K7D, 6K7E, 6K7F, 6K8Q - PubMed Abstract:
In protein crystals, flexible loops are frequently deformed by crystal contacts, whereas in solution, the large motions result in the poor convergence of such flexible loops in NMR structure determinations. We need an experimental technique to characterize the structural and dynamic properties of intrinsically flexible loops of protein molecules.
Organizational Affiliation:
Division of Structural Biology, Medical Institute of Bioregulation, Kyushu University, Maidashi 3-1-1, Higashi-ku, Fukuoka 812-8582, Japan.