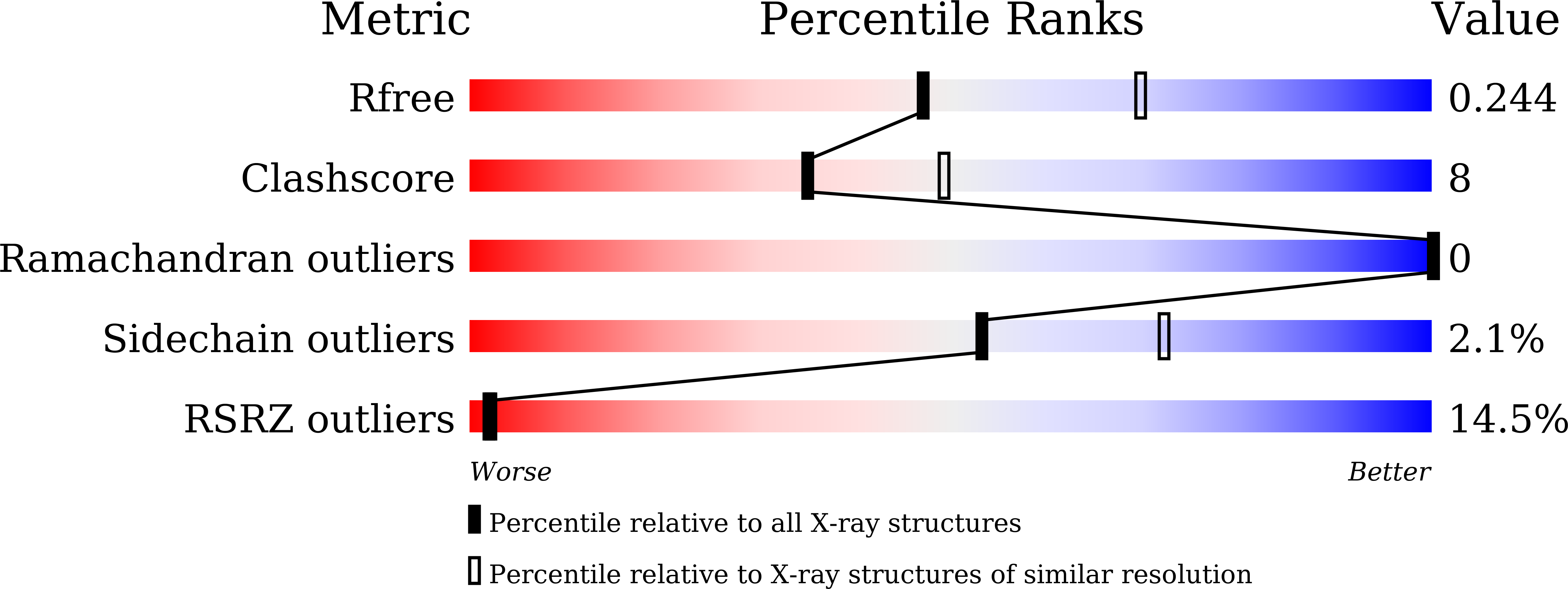
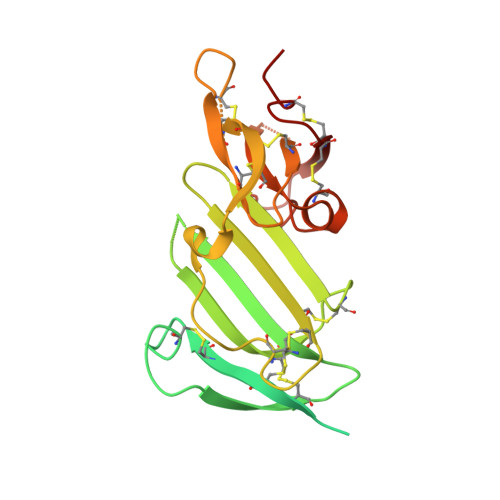
Crystal structure of the unoccupied murine urokinase-type plasminogen activator receptor (uPAR) reveals a tightly packed DII-DIII unit.
Liu, M., Lin, L., Hoyer-Hansen, G., Ploug, M., Li, H., Jiang, L., Yuan, C., Li, J., Huang, M.(2019) FEBS Lett 593: 1236-1247
- PubMed: 31044429
- DOI: https://doi.org/10.1002/1873-3468.13397
- Primary Citation of Related Structures:
6AEX - PubMed Abstract:
The urokinase-type plasminogen activator receptor (uPAR) is a cell surface receptor that is capable of binding to a range of extracellular proteins and triggering a series of proteolytic and signaling events. Previous structural studies of uPAR with its ligands uPA and vitronectin revealed that its three domains (DI, DII, and DIII) form a large hydrophobic cavity to accommodate uPA. In the present study, the structure of unoccupied murine uPAR (muPAR) is determined. The structure of DII and DIII of muPAR is well defined and forms a compact globular unit, while DI could not be traced. Molecular dynamic simulations further confirm the rigid binding interface between DII and DIII. This study shows overall structural flexibility of uPAR in the absence of uPA.
Organizational Affiliation:
College of Biological Science and Engineering, Fuzhou University, China.