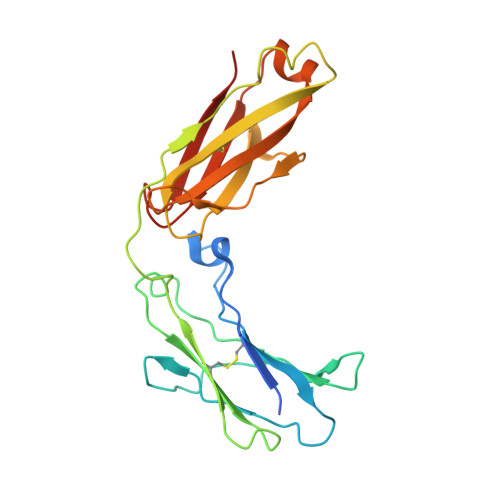
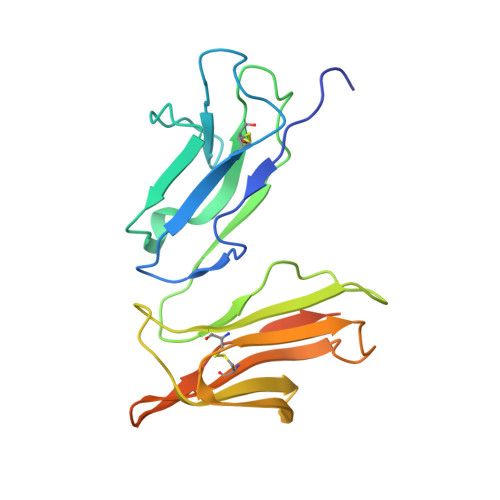
Decoding human-macaque interspecies differences in Fc-effector functions: The structural basis for CD16-dependent effector function in Rhesus macaques.
Tolbert, W.D., Gohain, N., Kremer, P.G., Hederman, A.P., Nguyen, D.N., Van, V., Sherburn, R., Lewis, G.K., Finzi, A., Pollara, J., Ackerman, M.E., Barb, A.W., Pazgier, M.(2022) Front Immunol 13: 960411-960411
- PubMed: 36131913
- DOI: https://doi.org/10.3389/fimmu.2022.960411
- Primary Citation of Related Structures:
6MJ3, 6MJO, 7KCZ - PubMed Abstract:
Fc mediated effector functions of antibodies play important roles in immunotherapies and vaccine efficacy but assessing those functions in animal models can be challenging due to species differences. Rhesus macaques, Macaca mulatta (Mm) share approximately 93% sequence identity with humans but display important differences in their adaptive immune system that complicates their use in validating therapeutics and vaccines that rely on Fc effector functions. In contrast to humans, macaques only have one low affinity FcγRIII receptor, CD16, which shares a polymorphism at position 158 with human FcγRIIIa with Ile 158 and Val 158 variants. Here we describe structure-function relationships of the Ile/Val 158 polymorphism in Mm FcγRIII. Our data indicate that the affinity of the allelic variants of Mm FcγRIII for the macaque IgG subclasses vary greatly with changes in glycan composition both on the Fc and the receptor. However, unlike the human Phe/Val 158 polymorphism in FcγRIIIa, the higher affinity variant corresponds to the larger, more hydrophobic side chain, Ile, even though it is not directly involved in the binding interface. Instead, this side chain appears to modulate glycan-glycan interactions at the Fc/FcγRIII interface. Furthermore, changes in glycan composition on the receptor have a greater effect for the Val 158 variant such that with oligomannose type glycans and with glycans only on Asn 45 and Asn 162 , Val 158 becomes the variant with higher affinity to Fc. These results have implications not only for the better interpretation of nonhuman primate studies but also for studies performed with human effector cells carrying different FcγRIIIa alleles.
Organizational Affiliation:
Infectious Disease Division, Department of Medicine, Uniformed Services University of the Health Sciences, Bethesda, MD, United States.