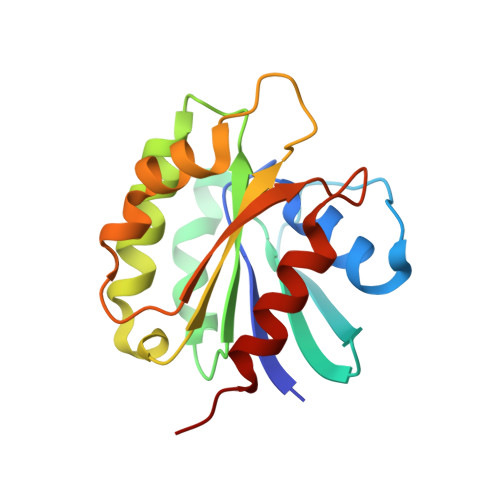
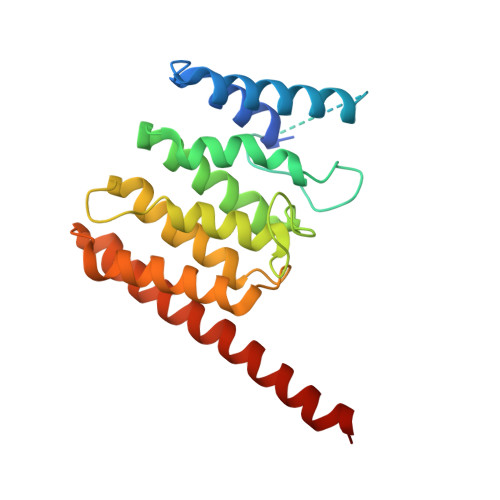
Structural basis for targeting BIG1 to Golgi apparatus through interaction of its DCB domain with Arl1
Wang, R., Wang, Z., Wang, K., Zhang, T., Ding, J.(2016) J Mol Cell Biol
- PubMed: 27436755
- DOI: https://doi.org/10.1093/jmcb/mjw033
- Primary Citation of Related Structures:
5J5C
Organizational Affiliation:
National Center for Protein Science Shanghai, State Key Laboratory of Molecular Biology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, 320 Yueyang Road, Shanghai200031, China.