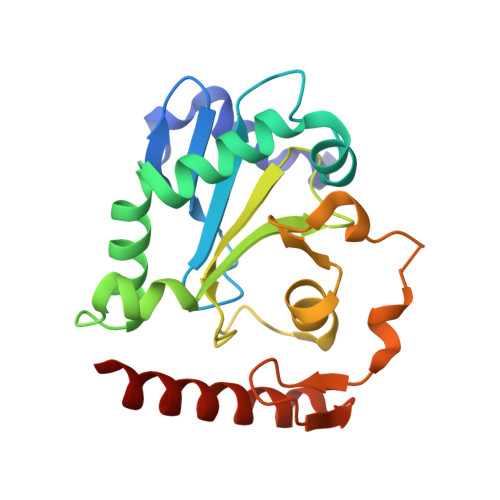
DNA3'pp5'G de-capping activity of aprataxin: effect of cap nucleoside analogs and structural basis for guanosine recognition.
Chauleau, M., Jacewicz, A., Shuman, S.(2015) Nucleic Acids Res 43: 6075-6083
- PubMed: 26007660
- DOI: https://doi.org/10.1093/nar/gkv501
- Primary Citation of Related Structures:
4XBA, 4YKL - PubMed Abstract:
DNA3'pp5'G caps synthesized by the 3'-PO4/5'-OH ligase RtcB have a strong impact on enzymatic reactions at DNA 3'-OH ends. Aprataxin, an enzyme that repairs A5'pp5'DNA ends formed during abortive ligation by classic 3'-OH/5'-PO4 ligases, is also a DNA 3' de-capping enzyme, converting DNAppG to DNA3'p and GMP. By taking advantage of RtcB's ability to utilize certain GTP analogs to synthesize DNAppN caps, we show that aprataxin hydrolyzes inosine and 6-O-methylguanosine caps, but is not adept at removing a deoxyguanosine cap. We report a 1.5 Å crystal structure of aprataxin in a complex with GMP, which reveals that: (i) GMP binds at the same position and in the same anti nucleoside conformation as AMP; and (ii) aprataxin makes more extensive nucleobase contacts with guanine than with adenine, via a hydrogen bonding network to the guanine O6, N1, N2 base edge. Alanine mutations of catalytic residues His147 and His149 abolish DNAppG de-capping activity, suggesting that the 3' de-guanylylation and 5' de-adenylylation reactions follow the same pathway of nucleotidyl transfer through a covalent aprataxin-(His147)-NMP intermediate. Alanine mutation of Asp63, which coordinates the guanosine ribose hydroxyls, impairs DNAppG de-capping.
Organizational Affiliation:
Molecular Biology Program, Sloan-Kettering Institute, New York, NY 10065, USA.