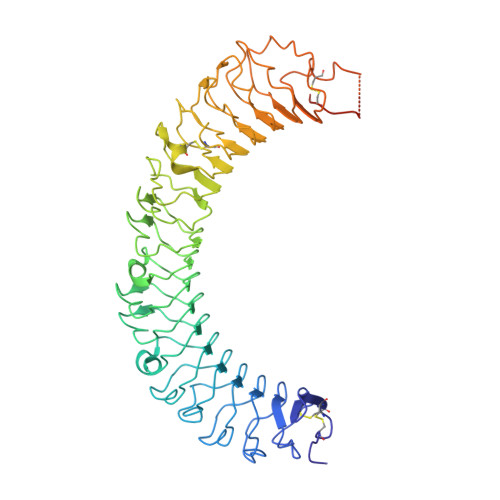
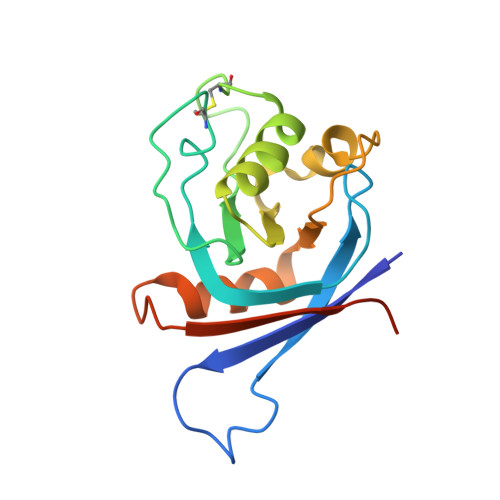
The structural basis of R-spondin recognition by LGR5 and RNF43.
Chen, P.H., Chen, X., Lin, Z., Fang, D., He, X.(2013) Genes Dev 27: 1345-1350
- PubMed: 23756651
- DOI: https://doi.org/10.1101/gad.219915.113
- Primary Citation of Related Structures:
4KNG - PubMed Abstract:
R-spondins (RSPOs) enhance Wnt signaling, affect stem cell behavior, bind to leucine-rich repeat-containing G-protein-coupled receptors 4-6, (LGR4-6) and the transmembrane E3 ubiquitin ligases RING finger 43/zinc and RING finger 3 (RNF43/ZNRF3). The structure of RSPO1 bound to both LGR5 and RNF43 ectodomains confirms their physical linkage. RSPO1 is sandwiched by LGR5 and RNF43, with its rod module of the cysteine-rich domain (CRD) contacting LGR5 and a hairpin inserted into RNF43. LGR5 does not contact RNF43 but increases the affinity of RSPO1 to RNF43, supporting LGR5 as an engagement receptor and RNF43 as an effector receptor. Disease mutations map to the RSPO1-RNF43 interface, which promises therapeutic targeting.
Organizational Affiliation:
Department of Molecular Pharmacology and Biological Chemistry, Northwestern University Feinberg School of Medicine, Chicago, IL 60611, USA.