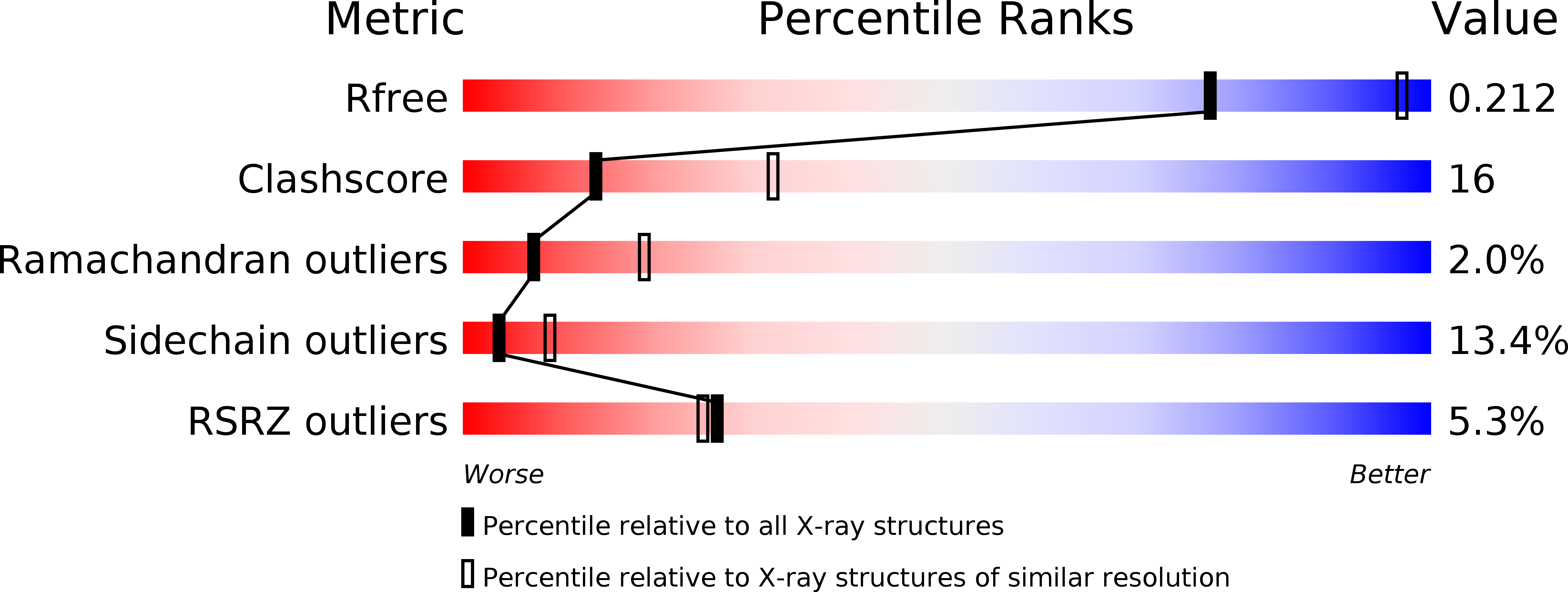
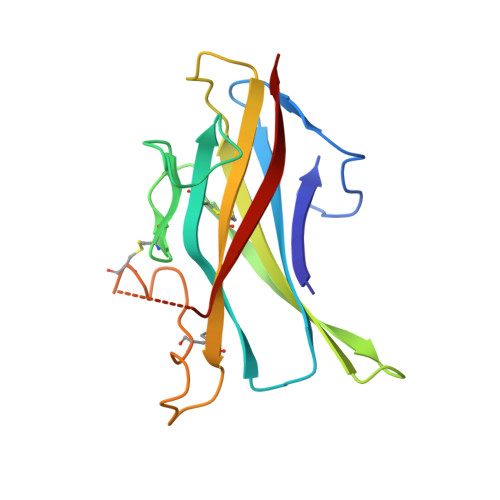
Identification of a Novel Tgf-Beta-Binding Site in the Zona Pellucida C-Terminal (Zp-C) Domain of Tgf-Beta-Receptor-3 (Tgfr-3).
Diestel, U., Resch, M., Meinhardt, K., Weiler, S., Hellmann, T.V., Mueller, T.D., Nickel, J., Eichler, J., Muller, Y.A.(2013) PLoS One 8: 67214
- PubMed: 23826237
- DOI: https://doi.org/10.1371/journal.pone.0067214
- Primary Citation of Related Structures:
4AJV - PubMed Abstract:
The zona pellucida (ZP) domain is present in extracellular proteins such as the zona pellucida proteins and tectorins and participates in the formation of polymeric protein networks. However, the ZP domain also occurs in the cytokine signaling co-receptor transforming growth factor β (TGF-β) receptor type 3 (TGFR-3, also known as betaglycan) where it contributes to cytokine ligand recognition. Currently it is unclear how the ZP domain architecture enables this dual functionality. Here, we identify a novel major TGF-β-binding site in the FG loop of the C-terminal subdomain of the murine TGFR-3 ZP domain (ZP-C) using protein crystallography, limited proteolysis experiments, surface plasmon resonance measurements and synthetic peptides. In the murine 2.7 Å crystal structure that we are presenting here, the FG-loop is disordered, however, well-ordered in a recently reported homologous rat ZP-C structure. Surprisingly, the adjacent external hydrophobic patch (EHP) segment is registered differently in the rat and murine structures suggesting that this segment only loosely associates with the remaining ZP-C fold. Such a flexible and temporarily-modulated association of the EHP segment with the ZP domain has been proposed to control the polymerization of ZP domain-containing proteins. Our findings suggest that this flexibility also extends to the ZP domain of TGFR-3 and might facilitate co-receptor ligand interaction and presentation via the adjacent FG-loop. This hints that a similar C-terminal region of the ZP domain architecture possibly regulates both the polymerization of extracellular matrix proteins and cytokine ligand recognition of TGFR-3.
Organizational Affiliation:
Lehrstuhl fuer Biotechnik, Department of Biology, Friedrich-Alexander-University Erlangen-Nuremberg, Erlangen, Germany.