Crystal Structures of Starch Binding Domain from Rhizopus Oryzae Glucoamylase in Complex with Isomaltooligosaccharide: Insights Into Polysaccharide Binding Mechanism of Cbm21 Family.
Chu, C., Li, K., Lin, S., Chang, M.D., Jiang, T., Sun, Y.(2014) Proteins 82: 1079
- PubMed: 24108499
- DOI: https://doi.org/10.1002/prot.24446
- Primary Citation of Related Structures:
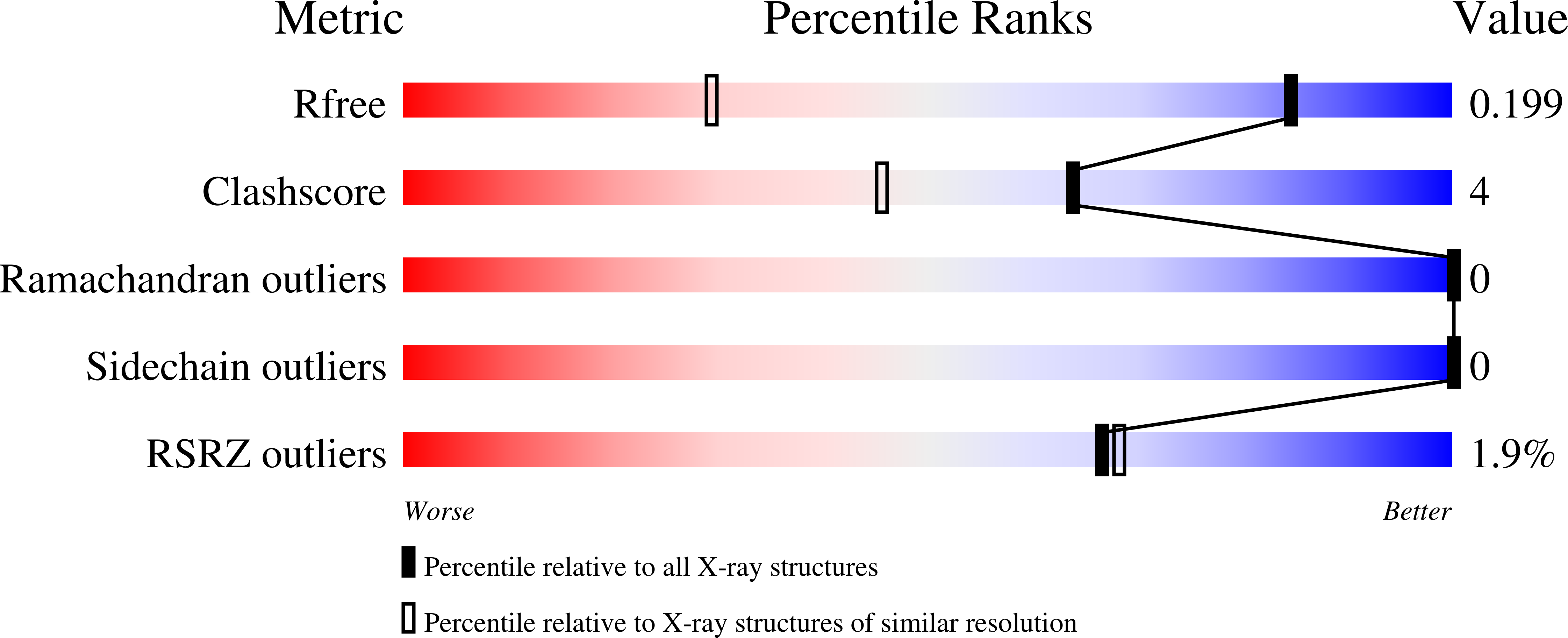
4BFN, 4BFO - PubMed Abstract:
Glucoamylases are responsible for hydrolysis of starch and polysaccharides to yield β-D-glucose. Rhizopus oryzae glucoamylase (RoGA) is composed of an N-terminal starch binding domain (SBD) and a C-terminal catalytic domain connected by an O-glycosylated linker. Two carbohydrate binding sites in RoSBD have been identified, site I is created by three highly conserved aromatic residues, Trp47, Tyr83, and Tyr94, and site II is built up by Tyr32 and Phe58. Here, the two crystal structures of RoSBD in complex with only α-(1,6)-linked isomaltotriose (RoSBD-isoG3) and isomaltotetraose (RoSBD-isoG4) have been determined at 1.2 and 1.3 Å, respectively. Interestingly, site II binding is observed in both complexes, while site I binding is only found in the RoSBD-isoG4 complex. Hence, site II acts as the recognition binding site for carbohydrate and site I accommodates site II to bind isoG4. Site I participates in sugar binding only when the number of glucosyl units of oligosaccharides is more than three. Taken together, two carbohydrate binding sites in RoSBD cooperate to reinforce binding mode of glucoamylase with polysaccharides as well as the starch.
Organizational Affiliation:
Department of Life Science and Institute of Bioinformatics and Structural Biology, College of Life Science, National Tsing Hua University, Hsin Chu, 30013, Taiwan, Republic of China.