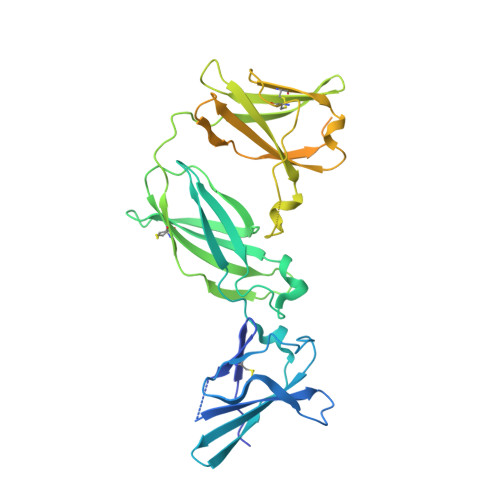
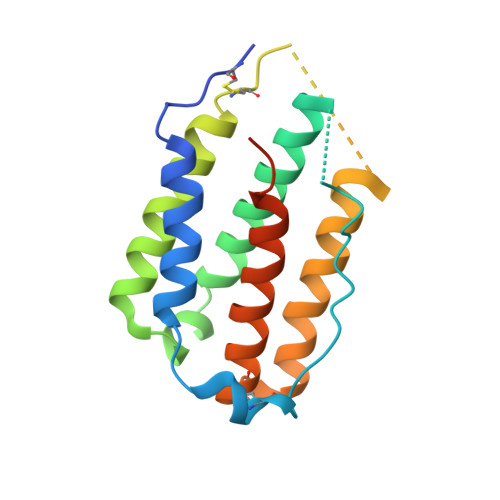
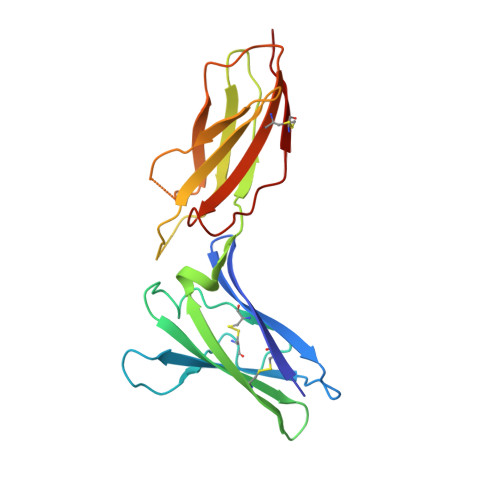
Structural linkage between ligand discrimination and receptor activation by type I interferons.
Thomas, C., Moraga, I., Levin, D., Krutzik, P.O., Podoplelova, Y., Trejo, A., Lee, C., Yarden, G., Vleck, S.E., Glenn, J.S., Nolan, G.P., Piehler, J., Schreiber, G., Garcia, K.C.(2011) Cell 146: 621-632
- PubMed: 21854986
- DOI: https://doi.org/10.1016/j.cell.2011.06.048
- Primary Citation of Related Structures:
3S8W, 3S98, 3S9D, 3SE3, 3SE4 - PubMed Abstract:
Type I Interferons (IFNs) are important cytokines for innate immunity against viruses and cancer. Sixteen human type I IFN variants signal through the same cell-surface receptors, IFNAR1 and IFNAR2, yet they can evoke markedly different physiological effects. The crystal structures of two human type I IFN ternary signaling complexes containing IFNα2 and IFNω reveal recognition modes and heterotrimeric architectures that are unique among the cytokine receptor superfamily but conserved between different type I IFNs. Receptor-ligand cross-reactivity is enabled by conserved receptor-ligand "anchor points" interspersed among ligand-specific interactions that "tune" the relative IFN-binding affinities, in an apparent extracellular "ligand proofreading" mechanism that modulates biological activity. Functional differences between IFNs are linked to their respective receptor recognition chemistries, in concert with a ligand-induced conformational change in IFNAR1, that collectively control signal initiation and complex stability, ultimately regulating differential STAT phosphorylation profiles, receptor internalization rates, and downstream gene expression patterns.
Organizational Affiliation:
Howard Hughes Medical Institute, Department of Molecular and Cellular Physiology, Stanford University School of Medicine, Stanford, CA 94305, USA.