Structural Insights into Novel Functions of a pro-survival PP2A-specific Methyltransferase
Stanevich, V., Jiang, L., Satyshur, K.A., Li, Y., Jeffrey, P.D., Li, Z., Semmelhack, M.F., Xing, Y.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Leucine carboxyl methyltransferase 1 | 334 | Homo sapiens | Mutation(s): 4 Gene Names: CGI-68, LCMT, LCMT-1, LCMT1 EC: 2.1.1 | 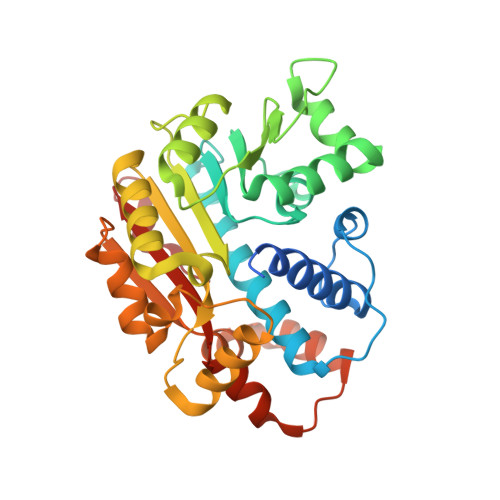 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9UIC8 (Homo sapiens) Explore Q9UIC8 Go to UniProtKB: Q9UIC8 | |||||
PHAROS: Q9UIC8 GTEx: ENSG00000205629 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9UIC8 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SAH Query on SAH | I [auth A] K [auth B] N [auth C] P [auth D] S [auth E] | S-ADENOSYL-L-HOMOCYSTEINE C14 H20 N6 O5 S ZJUKTBDSGOFHSH-WFMPWKQPSA-N |  | ||
| MES Query on MES | AA [auth H], L [auth B], Q [auth D], T [auth E] | 2-(N-MORPHOLINO)-ETHANESULFONIC ACID C6 H13 N O4 S SXGZJKUKBWWHRA-UHFFFAOYSA-N |  | ||
| GOL Query on GOL | BA [auth H] J [auth A] M [auth B] O [auth C] R [auth D] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 106.036 | α = 105.41 |
| b = 81.127 | β = 93.97 |
| c = 82.816 | γ = 104.29 |
| Software Name | Purpose |
|---|---|
| CBASS | data collection |
| PHASER | phasing |
| REFMAC | refinement |
| DENZO | data reduction |
| SCALEPACK | data scaling |