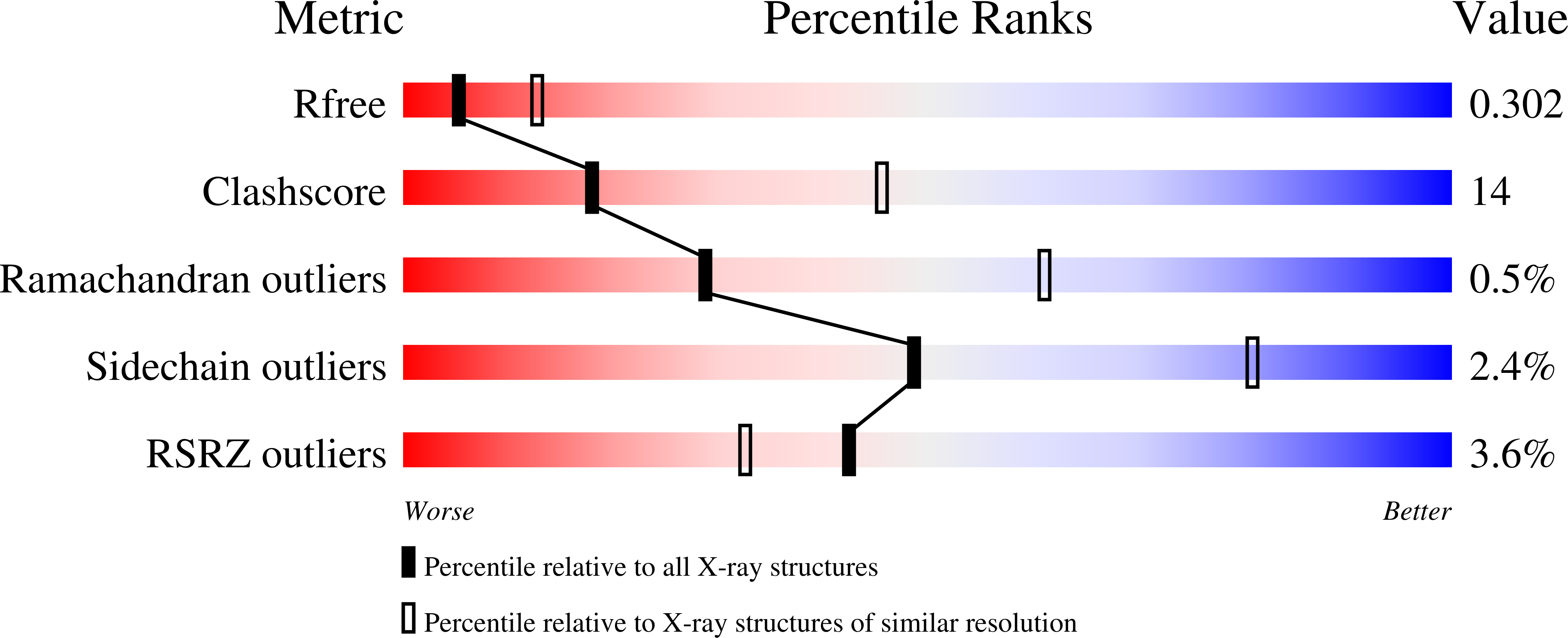
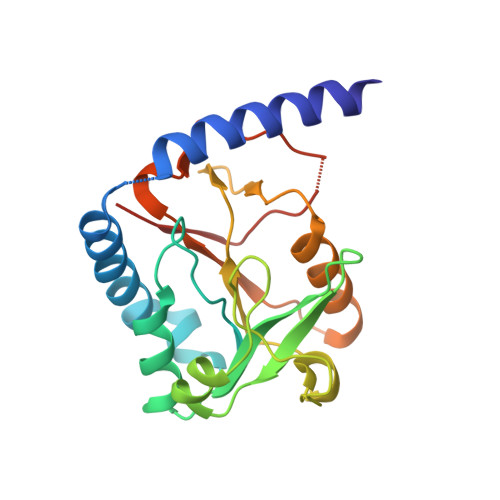
Structural basis for the inhibition of human 5,10-methenyltetrahydrofolate synthetase by N10-substituted folate analogues
Wu, D., Li, Y., Song, G., Cheng, C., Zhang, R., Joachimiak, A., Shaw, N., Liu, Z.-J.(2009) Cancer Res 69: 7294-7301
- PubMed: 19738041
- DOI: https://doi.org/10.1158/0008-5472.CAN-09-1927
- Primary Citation of Related Structures:
3HXT, 3HY3, 3HY4, 3HY6 - PubMed Abstract:
5,10-Methenyltetrahydrofolate synthetase (MTHFS) regulates the flow of carbon through the one-carbon metabolic network, which supplies essential components for the growth and proliferation of cells. Inhibition of MTHFS in human MCF-7 breast cancer cells has been shown to arrest the growth of cells. Absence of the three-dimensional structure of human MTHFS (hMTHFS) has hampered the rational design and optimization of drug candidates. Here, we report the structures of native hMTHFS, a binary complex of hMTHFS with ADP, hMTHFS bound with the N5-iminium phosphate reaction intermediate, and an enzyme-product complex of hMTHFS. The N5-iminium phosphate captured for the first time in our crystal structure unravels a unique strategy used by hMTHFS for recognition of the substrate and provides structural basis for the regulation of enzyme activity. Binding of N10-substituted folate analogues places Y152 in the middle of the channel connecting ATP binding site with the substrate binding pocket, precluding the positioning of gamma-phosphate for a nucleophilic attack. Using the structures of hMTHFS as a guide, we have probed the role of residues surrounding the active site in catalysis by mutagenesis. The ensemble of hMTHFS structures and the mutagenesis data yield a coherent picture of the MTHFS active site, determinants of substrate specificity, and new insights into the mechanism of inhibition of hMTHFS.
Organizational Affiliation:
National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China.