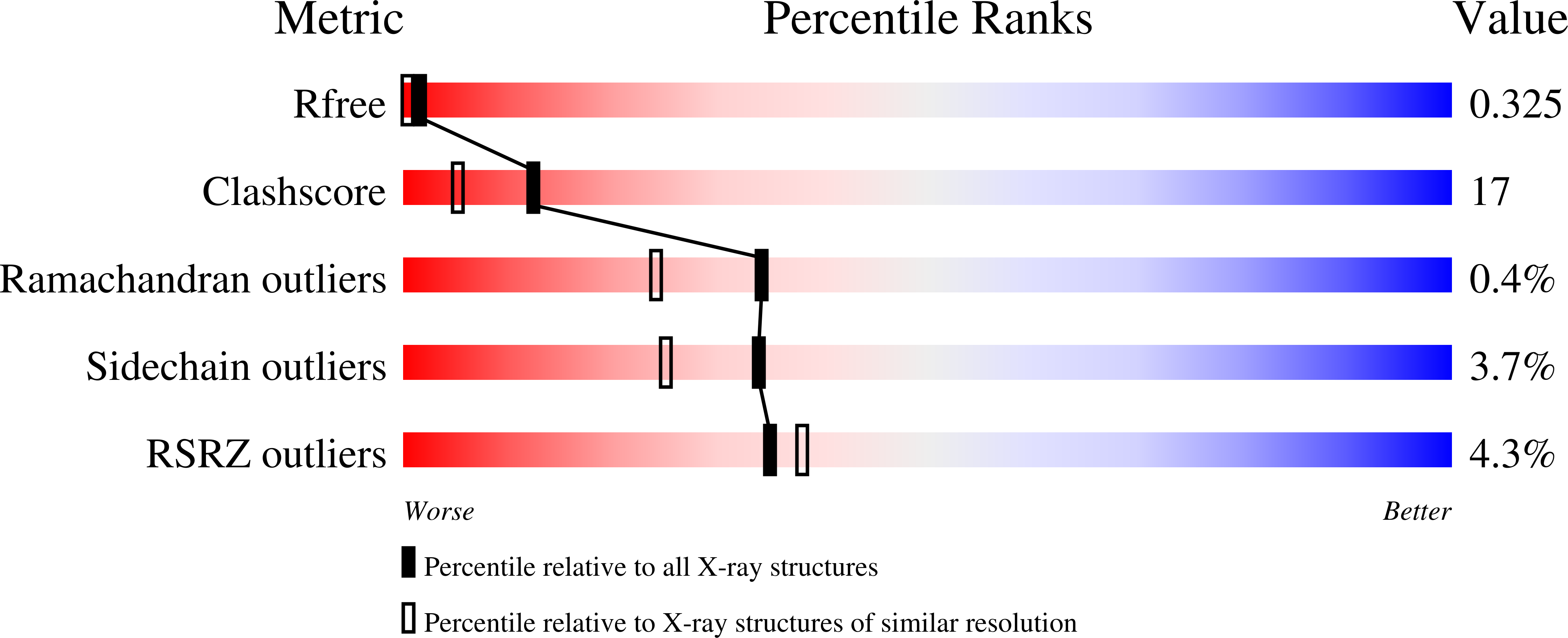
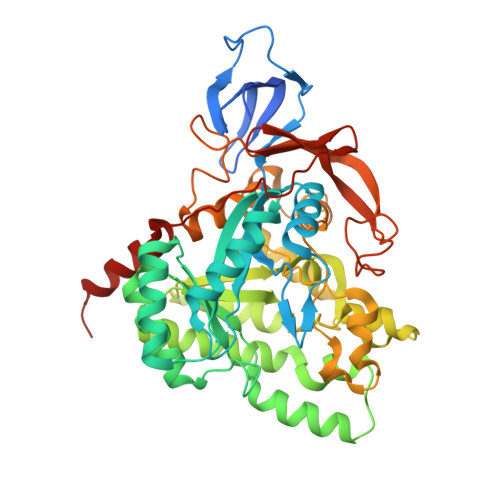
Crystal and Solution Structure, Stability and Post- Translational Modifications of Collapsin Response Mediator Protein 2.
Majava, V., Loytynoja, N., Chen, W.Q., Lubec, G., Kursula, P.(2008) FEBS J 275: 4583
- PubMed: 18699782
- DOI: https://doi.org/10.1111/j.1742-4658.2008.06601.x
- Primary Citation of Related Structures:
2VM8 - PubMed Abstract:
The collapsin response mediator protein 2 (CRMP-2) is a central molecule regulating axonal growth cone guidance. It interacts with the cytoskeleton and mediates signals related to myelin-induced axonal growth inhibition. CRMP-2 has also been characterized as a constituent of neurofibrillary tangles in Alzheimer's disease. CD spectroscopy and thermal stability assays using the Thermofluor method indicated that Ca2+ and Mg2+ affect the stability of CRMP-2 and prevent the formation of beta-aggregates upon heating. Gel filtration showed that the presence of Ca2+ or Mg2+ promoted the formation of CRMP-2 homotetramers, and this was further proven by small-angle X-ray scattering experiments, where a 3D solution structure for CRMP-2 was obtained. Previously, we described a crystal structure of human CRMP-2 complexed with calcium. In the present study, we determined the structure of CRMP-2 in the absence of calcium at 1.9 A resolution. When Ca2+ was omitted, crystals could only be grown in the presence of Mg2+ ions. By a proteomic approach, we further identified a number of post-translational modifications in CRMP-2 from rat brain hippocampus and mapped them onto the crystal structure.
Organizational Affiliation:
Department of Biochemistry, University of Oulu, Finland.