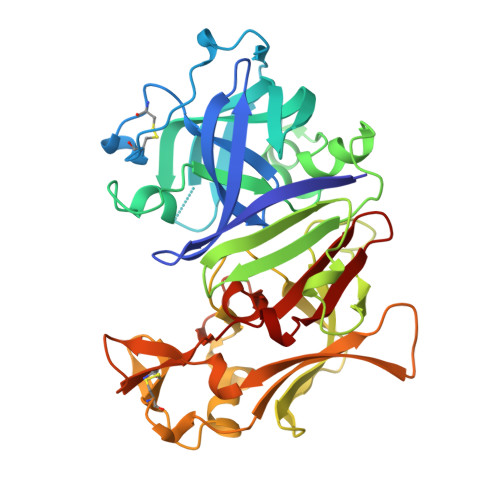
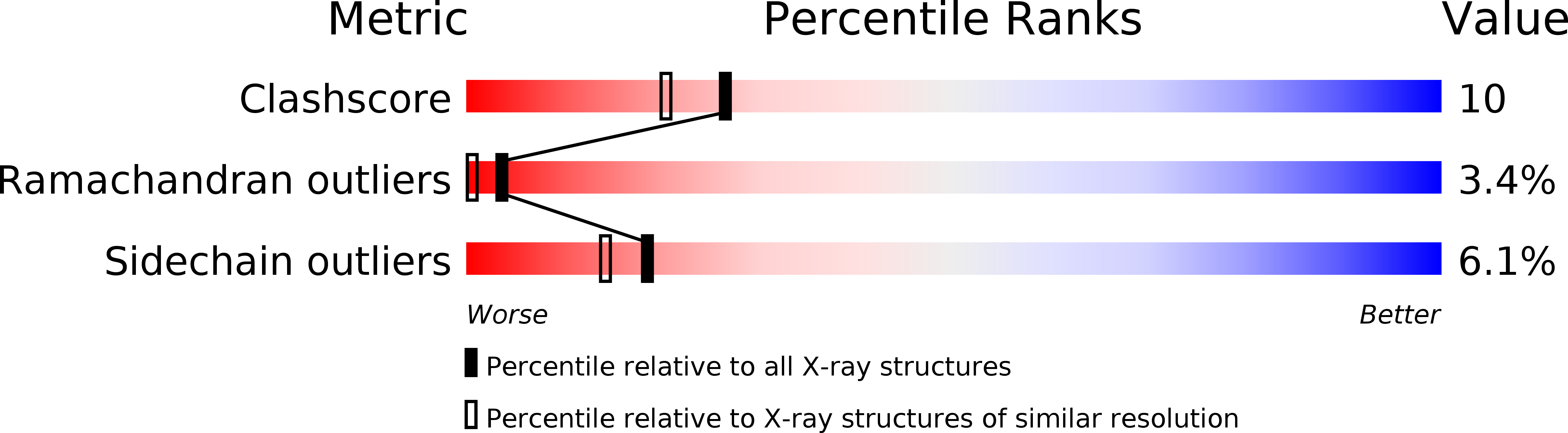Crystal structure of the aspartic proteinase from Rhizomucor miehei at 2.15 A resolution.
Yang, J., Teplyakov, A., Quail, J.W.(1997) J Mol Biol 268: 449-459
- PubMed: 9159482
- DOI: https://doi.org/10.1006/jmbi.1997.0968
- Primary Citation of Related Structures:
2ASI - PubMed Abstract:
The crystal structure of the aspartic proteinase from Rhizomucor miehei (RMP, EC 3. 4. 23. 23) has been refined to 2.15 A resolution to a crystallographic R-value of 0.215 and an Rfree of 0.281. The root-mean-square (r.m.s.) error for the atomic coordinates estimated from a Luzzati plot is 0.2 A. The r.m.s. deviations for the bond distances and bond angles from ideality are 0.01 A and 1.7 degrees, respectively. RMP contains two domains that consist predominantly of beta-sheets. A large substrate-binding cleft is clearly visible between the two domains, and the two catalytic residues Asp38 and Asp237 are located in the middle of the cleft with a water molecule bridging the carboxyl groups of Asp38 and Asp237. Due to crystal packing, the C-terminal domain is more mobile than the N-terminal domain. Most of the aspartic proteinases (except renin) reach their maximum activity at acidic pH. We propose that the optimum pH of each aspartic proteinase is determined by the electrostatic potential at the active site, which, in turn, is determined by the positions and orientations of all the residues near the active site. RMP is the most glycosylated among the aspartic proteinases. The carbohydrate moieties are linked to Asn79 and Asn188. Asn79 is in the middle of a beta-strand and Asn188 is on a surface loop in contrast to the previous hypothesis proposed by Brown and Yada that they are both on surface beta-turns. RMP has a very high thermal stability. The high thermal stability is probably due to the high level of glycosylation. We propose that the highly flexible carbohydrates act as heat reservoirs to stabilize the conformation of RMP and therefore give the enzyme a high level of thermal stability. Three-dimensional structural and sequence alignments of RMP with other aspartic proteinases show that RMP is most structurally homologous to that of Mucor pusillus (MPP), and differs from other fungal enzymes as much as it does from the mammalian enzymes. This suggests that RMP and MPP diverged from the main stream of aspartic proteinases at an early stage of evolution. The present study adds a second member to this subfamily of aspartic proteinases.
Organizational Affiliation:
Department of Chemistry, University of Saskatchewan, Saskatoon, Canada.