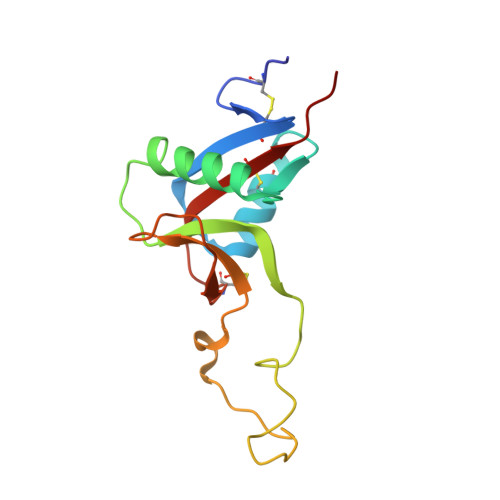
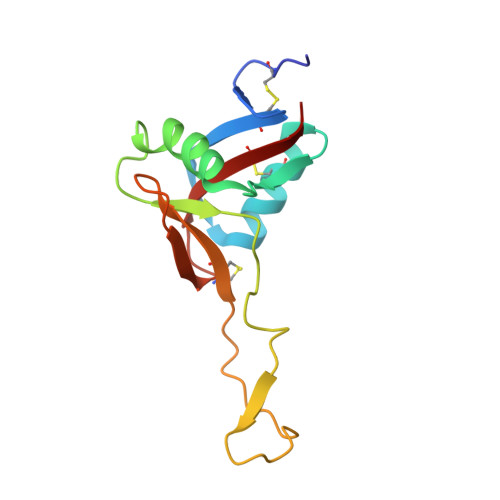
Crystal structure of a platelet-agglutinating factor isolated from the venom of Taiwan habu (Trimeresurus mucrosquamatus).
Huang, K.F., Ko, T.P., Hung, C.C., Chu, J., Wang, A.H., Chiou, S.H.(2004) Biochem J 378: 399-407
- PubMed: 14613481
- DOI: https://doi.org/10.1042/BJ20031507
- Primary Citation of Related Structures:
1V4L - PubMed Abstract:
Platelet glycoprotein Ib (GPIb)-binding proteins (GPIb-BPs) from snake venoms are usually C-type lectins, which target specific sites of GPIbalpha and elicit distinct effects on platelets. In the present paper, we report a tetrameric platelet-agglutinating factor (molecular mass 121.1 kDa), termed mucrocetin, purified from the venom of Taiwan habu (Trimeresurus mucrosquamatus ). Mucrocetin is a GPIbalpha agonist with a binding site distinct from that of flavocetin-A (a snake venom GPIbalpha antagonist) on GPIbalpha, in spite of the high sequence identity (94.6%) between the two venom lectins. The crystal structure of mucrocetin was solved and refined to 2.8 A (1 A=0.1 nm) resolution, which shows an interesting crystal packing of six-layer cylinders of doughnut-shaped molecules. The four alphabeta heterodimers are arranged in an unusual square-shaped ring stabilized by four interdimer 'head-to-tail' disulphide bridges. Detailed structural comparison between mucrocetin and flavocetin-A suggests that their disparate platelet effects are probably attributable to different charge distributions on the putative concave binding surface. A unique positively charged patch on the binding surface of mucrocetin, formed by Lys102, Lys108, Lys109 and Arg123 in the alpha-subunit coupled with Lys22, Lys102, Lys116 and Arg117 in the beta-subunit, appears to be the primary determinant of its platelet-agglutinating activity. Conceivably, this interesting venom factor may provide a useful tool to study platelet agglutination by binding to the GPIb-IX-V complex.
Organizational Affiliation:
Institute of Biological Chemistry, Academia Sinica, Nankang, Taipei 11529, Taiwan.