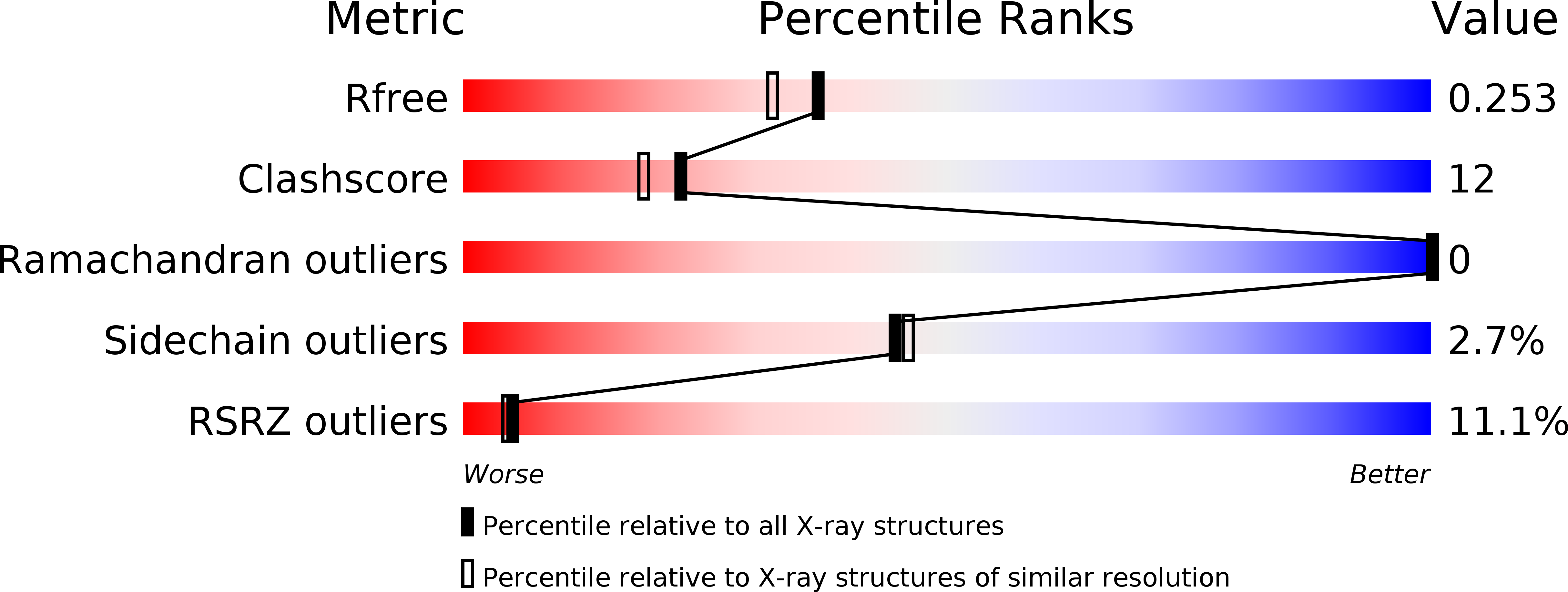
Structural characterization and kinetics of nitric-oxide synthase inhibition by novel N5-(iminoalkyl)- and N5-(iminoalkenyl)-ornithines
Bretscher, L.E., Li, H., Poulos, T.L., Griffith, O.W.(2003) J Biol Chem 278: 46789-46797
- PubMed: 12960153
- DOI: https://doi.org/10.1074/jbc.M306787200
- Primary Citation of Related Structures:
1MMV, 1MMW - PubMed Abstract:
Isoform-specific nitric-oxide synthase (NOS) inhibitors may prove clinically useful in reducing the pathophysiological effects associated with increased neuronal NOS (nNOS) or inducible NOS (iNOS) activity in a variety of neurological and inflammatory disorders. Analogs of the NOS substrate L-arginine are pharmacologically attractive inhibitors because of their stability, reliable cell uptake, and good selectivity for NOS over other heme proteins. Some inhibitory arginine analogs show significant isoform selectivity although the structural or mechanistic basis of such selectivity is generally poorly understood. In the present studies, we determined by x-ray crystallography the binding interactions between rat nNOS and N5-(1-imino-3-butenyl)-L-ornithine (L-VNIO), a previously identified mechanism-based, irreversible inactivator with moderate nNOS selectivity. We have also synthesized and mechanistically characterized several L-VNIO analogs and find, surprisingly, that even relatively minor structural changes produce inhibitors that are either iNOS-selective or non-selective. Furthermore, derivatives having a methyl group added to the butenyl moiety of L-VNIO and L-VNIO derivatives that are analogs of homoarginine rather than arginine display slow-on, slow-off kinetics rather than irreversible inactivation. These results elucidate some of the structural requirements for isoform-selective inhibition by L-VNIO and its related alkyl- and alkenyl-imino ornithine and lysine derivatives and may provide information useful in the ongoing rational design of isoform-selective inhibitors.
Organizational Affiliation:
Department of Biochemistry, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226, USA.