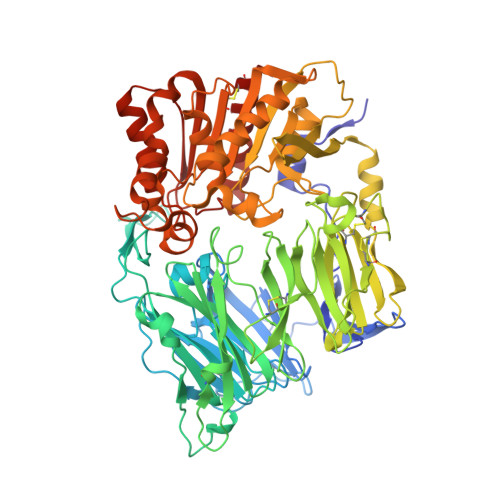
High-resolution structure of human apo dipeptidyl peptidase IV/CD26 and its complex with 1-[([2-[(5-iodopyridin-2-yl)amino]-ethyl]amino)-acetyl]-2-cyano-(S)-pyrrolidine.
Oefner, C., D'Arcy, A., Mac Sweeney, A., Pierau, S., Gardiner, R., Dale, G.E.(2003) Acta Crystallogr D Biol Crystallogr 59: 1206-1212
- PubMed: 12832764
- DOI: https://doi.org/10.1107/s0907444903010059
- Primary Citation of Related Structures:
1PFQ - PubMed Abstract:
Dipeptidyl peptidase IV is a multifunctional type II transmembrane serine protease glycoprotein. The high-resolution crystal structure of the homodimeric human apo dipeptidyl peptidase IV has been determined at 1.9 A resolution. In addition, the structure of the binary complex with 1-[([2-[(5-iodopyridin-2-yl)amino]-ethyl]amino)-acetyl]-2-cyano-(S)-pyrrolidine has been solved, revealing the nature of the covalent interaction with the active-site serine.
Organizational Affiliation:
Morphochem AG, Basel, Switzerland. christian.oefner@morphochem.ch