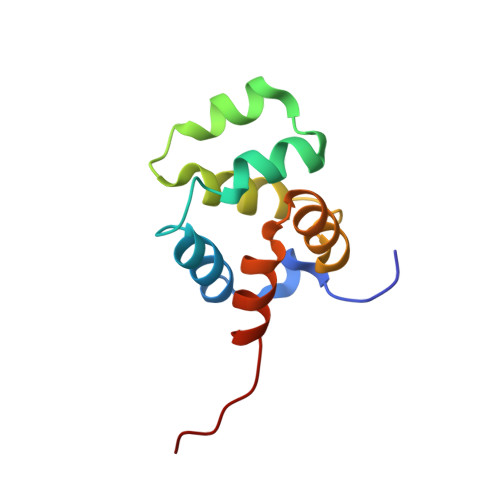
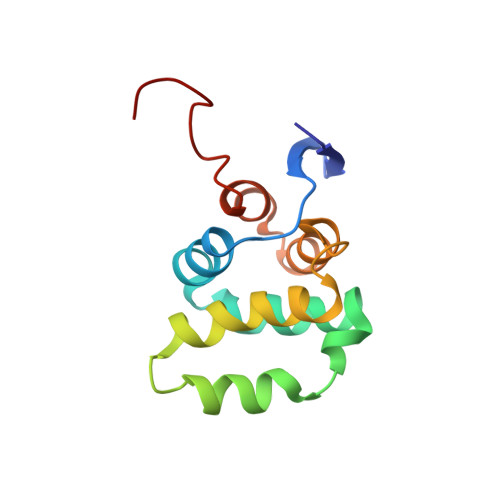
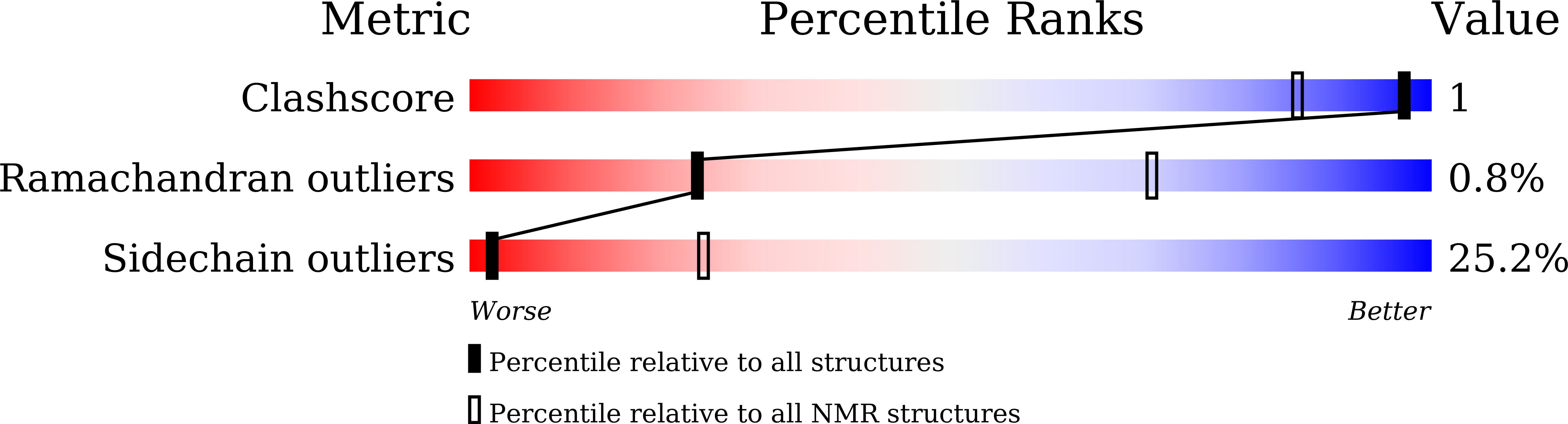Structural basis of NF-kappa B signaling by the p75 neurotrophin receptor interaction with adaptor protein TRADD through their respective death domains.
Zhang, N., Kisiswa, L., Ramanujan, A., Li, Z., Sim, E.W., Tian, X., Yuan, W., Ibanez, C.F., Lin, Z.(2021) J Biol Chem 297: 100916-100916
- PubMed: 34175311
- DOI: https://doi.org/10.1016/j.jbc.2021.100916
- Primary Citation of Related Structures:
7CSQ - PubMed Abstract:
The p75 neurotrophin receptor (p75 NTR ) is a critical mediator of neuronal death and tissue remodeling and has been implicated in various neurodegenerative diseases and cancers. The death domain (DD) of p75 NTR is an intracellular signaling hub and has been shown to interact with diverse adaptor proteins. In breast cancer cells, binding of the adaptor protein TRADD to p75 NTR depends on nerve growth factor and promotes cell survival. However, the structural mechanism and functional significance of TRADD recruitment in neuronal p75 NTR signaling remain poorly understood. Here we report an NMR structure of the p75 NTR -DD and TRADD-DD complex and reveal the mechanism of specific recognition of the TRADD-DD by the p75 NTR -DD mainly through electrostatic interactions. Furthermore, we identified spatiotemporal overlap of p75 NTR and TRADD expression in developing cerebellar granule neurons (CGNs) at early postnatal stages and discover the physiological relevance of the interaction between TRADD and p75 NTR in the regulation of canonical NF-κB signaling and cell survival in CGNs. Our results provide a new structural framework for understanding how the recruitment of TRADD to p75 NTR through DD interactions creates a membrane-proximal platform, which can be efficiently regulated by various neurotrophic factors through extracellular domains of p75 NTR , to propagate downstream signaling in developing neurons.
Organizational Affiliation:
School of Life Sciences, Tianjin University, Tianjin, PR China; Tianjin Key Laboratory of Function and Application of Biological Macromolecular Structures, School of Life Sciences, Tianjin University, Tianjin, PR China.