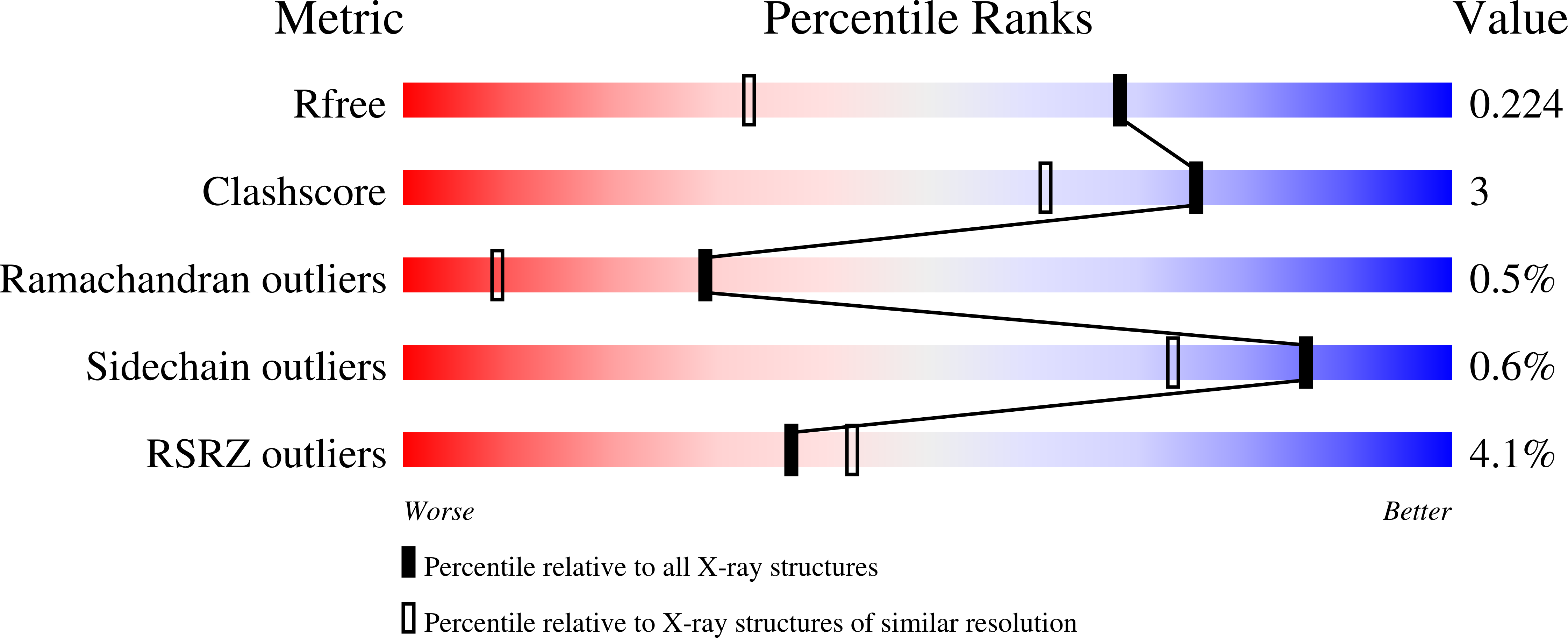
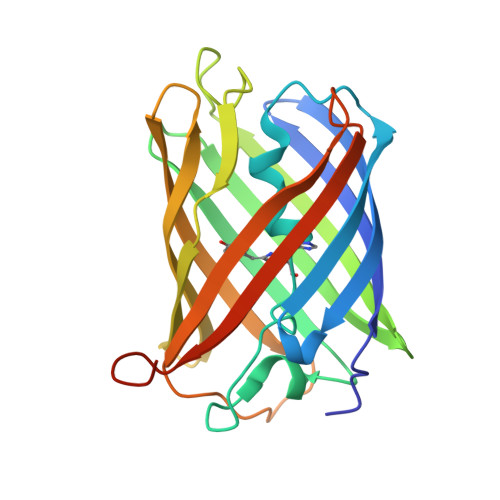
Directionality of light absorption and emission in representative fluorescent proteins.
Myskova, J., Rybakova, O., Brynda, J., Khoroshyy, P., Bondar, A., Lazar, J.(2020) Proc Natl Acad Sci U S A 117: 32395-32401
- PubMed: 33273123
- DOI: https://doi.org/10.1073/pnas.2017379117
- Primary Citation of Related Structures:
6YLM, 6YLN, 6YLO, 6YLP, 6YLQ, 6YLS - PubMed Abstract:
Fluorescent molecules are like antennas: The rate at which they absorb light depends on their orientation with respect to the incoming light wave, and the apparent intensity of their emission depends on their orientation with respect to the observer. However, the directions along which the most important fluorescent molecules in biology, fluorescent proteins (FPs), absorb and emit light are generally not known. Our optical and X-ray investigations of FP crystals have now allowed us to determine the molecular orientations of the excitation and emission transition dipole moments in the FPs mTurquoise2, eGFP, and mCherry, and the photoconvertible FP mEos4b. Our results will allow using FP directionality in studies of molecular and biological processes, but also in development of novel bioengineering and bioelectronics applications.
Organizational Affiliation:
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, 16610 Prague 6, Czech Republic.